IndoxacarbCAS# 144171-61-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144171-61-9 | SDF | Download SDF |
| PubChem ID | 9936739 | Appearance | Cryst. |
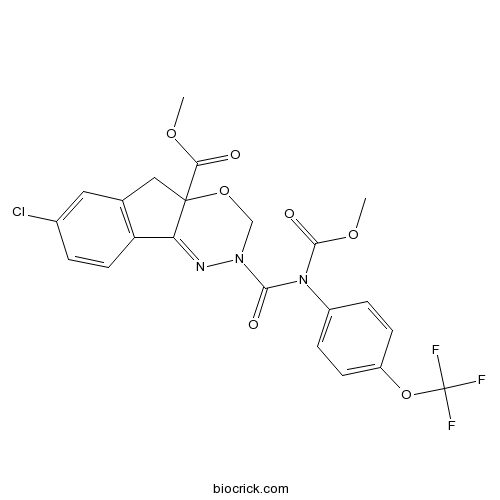
| Formula | C22H17ClF3N3O7 | M.Wt | 527.83 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl 7-chloro-2-[methoxycarbonyl-[4-(trifluoromethoxy)phenyl]carbamoyl]-3,5-dihydroindeno[1,2-e][1,3,4]oxadiazine-4a-carboxylate | ||
| SMILES | COC(=O)C12CC3=C(C1=NN(CO2)C(=O)N(C4=CC=C(C=C4)OC(F)(F)F)C(=O)OC)C=CC(=C3)Cl | ||
| Standard InChIKey | VBCVPMMZEGZULK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H17ClF3N3O7/c1-33-18(30)21-10-12-9-13(23)3-8-16(12)17(21)27-28(11-35-21)19(31)29(20(32)34-2)14-4-6-15(7-5-14)36-22(24,25)26/h3-9H,10-11H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Given Indoxacarb's effect on adult fleas, egg production and egg viability, this formulation can interrupt flea reproduction on treated cats for at least 6 weeks after treatment. |
| Targets | Antifection |

Indoxacarb Dilution Calculator

Indoxacarb Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8945 mL | 9.4727 mL | 18.9455 mL | 37.891 mL | 47.3637 mL |
| 5 mM | 0.3789 mL | 1.8945 mL | 3.7891 mL | 7.5782 mL | 9.4727 mL |
| 10 mM | 0.1895 mL | 0.9473 mL | 1.8945 mL | 3.7891 mL | 4.7364 mL |
| 50 mM | 0.0379 mL | 0.1895 mL | 0.3789 mL | 0.7578 mL | 0.9473 mL |
| 100 mM | 0.0189 mL | 0.0947 mL | 0.1895 mL | 0.3789 mL | 0.4736 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ledipasvir acetone
Catalog No.:BCC4046
CAS No.:1441674-54-9
- Eprosartan Mesylate
Catalog No.:BCC4658
CAS No.:144143-96-4
- Fmoc-Glu-OAll
Catalog No.:BCC3490
CAS No.:144120-54-7
- Fmoc-Asp-OAll
Catalog No.:BCC3086
CAS No.:144120-53-6
- Tarasaponin VII
Catalog No.:BCN2684
CAS No.:144118-18-3
- Deltarasin hydrochloride
Catalog No.:BCC4270
CAS No.:1440898-82-7
- Deltarasin
Catalog No.:BCC1524
CAS No.:1440898-61-2
- Febuxostat
Catalog No.:BCC2556
CAS No.:144060-53-7
- 6-O-Syringoylajugol
Catalog No.:BCN6246
CAS No.:144049-72-9
- Piclamilast
Catalog No.:BCC6215
CAS No.:144035-83-6
- BRD73954
Catalog No.:BCC5652
CAS No.:1440209-96-0
- Sodium Nitroprusside
Catalog No.:BCC4844
CAS No.:14402-89-2
- 1-Acetyl-4-methylpiperazine hydrochloride
Catalog No.:BCC6615
CAS No.:144205-68-5
- YYA-021
Catalog No.:BCC5346
CAS No.:144217-65-2
- Dehydroadynerigenin glucosyldigitaloside
Catalog No.:BCN1568
CAS No.:144223-70-1
- ML 337
Catalog No.:BCC6345
CAS No.:1443118-44-2
- 1-Deazaadenosine
Catalog No.:BCC6204
CAS No.:14432-09-8
- Trilepisflavan
Catalog No.:BCN6786
CAS No.:1443218-16-3
- YM 26734
Catalog No.:BCC7396
CAS No.:144337-18-8
- Nemoralisin C
Catalog No.:BCN7680
CAS No.:1443421-84-8
- CCG 203971
Catalog No.:BCC5601
CAS No.:1443437-74-8
- GSK2838232
Catalog No.:BCC6372
CAS No.:1443461-21-9
- 8-(7-Hydroxy-3,7-dimethyl-2,5-octadienyloxy)psoralen
Catalog No.:BCN1567
CAS No.:144398-34-5
- 3-Oxopomolic acid methyl ester
Catalog No.:BCN3723
CAS No.:14440-23-4
Enantioselective degradation of indoxacarb from different commercial formulations applied to tea.[Pubmed:25644775]
Chirality. 2015 Mar;27(3):262-7.
The stereoselective degradation of Indoxacarb enriched with (+)-S-Indoxacarb (S/R:70/30) was investigated in three typical green teas. A convenient and precise chiral method was developed and validated for measuring Indoxacarb enantiomers in green tea. The developed method was based on high-performance liquid chromatography coupled with tandem mass spectrometry using a Chiralpak IC column. The stereoselective degradation of Indoxacarb enantiomers showed that the (+)-S-enantiomer dissipated faster than the (-)-R-enantiomer in all three typical tea farms. However, no enantiomerization was observed after applying pure (+)-S-Indoxacarb. Residues on tea plant of the active ingredient (+)-S-Indoxacarb from suspension concentrate (SC) was more persistent than that from emulsifiable concentrate (EC).
Enantiomeric separation of indoxacarb on an amylose-based chiral stationary phase and its application in study of indoxacarb degradation in water.[Pubmed:24687873]
Biomed Chromatogr. 2014 Oct;28(10):1371-7.
Direct semipreparative enantioseparation of Indoxacarb was performed on a semipreparative Chiralpak IA column using normal-phase high-performance liquid chromatography (HPLC) with n-hexane-isopropanol-ethyl acetate (70:20:10) mixture as mobile phase. Degradation of Indoxacarb (2.33S + 1R) and its two enantiopure isoforms in three aqueous buffer solutions and four water samples collected from natural water sources was then elucidated by HPLC analysis on Chiralpak IA column. Degradation of all three Indoxacarbs complied with first-order kinetics and demonstrated linearity with regression coefficients R(2) > n0.88. Indoxacarb (2.33S + 1R) underwent enantioselective degradation in river water, rain water, and buffer solution of pH 7.0. Enantiopure S-(+)-Indoxacarb and R-(-)-Indoxacarb were both found to be configurationally stable in water.
Comparative study of the selective degradations of two enantiomers in the racemate and an enriched concentration of indoxacarb in soils.[Pubmed:25134952]
J Agric Food Chem. 2014 Sep 17;62(37):9066-72.
In this study, selective degradations of the two enantiomers of Indoxacarb in the concentrate (2.33S/1R) and racemate (1S/1R) are examined. The absolute configurations of Indoxacarb enantiomers were determined using X-ray diffraction. The results showed that in two alkaline soils, the S-(+)-Indoxacarb was preferentially degraded in both the concentrate and racemate. In one acid soil, the two enantiomers degraded no-selectivity. In another acid soil and one neutral soil, the R-(-)-Indoxacarb was preferentially degraded in both the concentrate and racemate. Indoxacarb enantiomers were configurationally stable in the five soils, and no interconversion was observed during the incubation. Because no significant difference in degradation was observed after samples were sterilized, the observed enantioselectivity may be attributed primarily to microbial activity in soils. The results indicate that the selective degradation behavior was the same for both formulations that were tested.
Efficacy of indoxacarb applied to cats against the adult cat flea, Ctenocephalides felis, flea eggs and adult flea emergence.[Pubmed:23642104]
Parasit Vectors. 2013 May 3;6:126.
BACKGROUND: A study was conducted to evaluate the effect of Indoxacarb applied to cats on adult cat fleas, Ctenocephalides felis, flea egg production and adult flea emergence. METHODS: Sixteen cats were selected for the study and allocated to two treatment groups. Eight cats were treated with a 19.5% w/v topical spot-on solution of Indoxacarb on day 0 and eight cats served as untreated controls. Each cat was infested with 50 fleas on Days -2, 7, 14, 21, 28, 35 and 42. On Days 1, 2, and 3, and at 2 and 3 days after each post treatment reinfestation flea eggs were collected from the pan under each cat cage. Eggs were counted and viability assessed by evaluating adult flea emergence 28 days after egg collection. Three days after treatment or infestation, each cat was combed to remove and count live fleas. RESULTS: Treatment with Indoxacarb provided 100% efficacy following infestations on day -2, 7, 14, 21 and 28 and efficacy was 99.6% following infestations on days 35 and 42. Egg production from Indoxacarb treated cats was reduced by 99.9% within 72 hours of treatment. For subsequent infestations no eggs were produced from treated cats from day 8 through day 30. Egg production was still reduced by >/=95.8% through day 45. Indoxacarb treatment also reduced adult flea emergence from eggs for 5 weeks after treatment. The combination of reduction in egg numbers and egg viability from Indoxacarb treated cats reduced predicted flea emergence by 100% from days 2 - 31 and 99.9%, 100%, 96.4% and 99.0% on days 37, 38, 44 and 45, respectively. CONCLUSIONS: A topical spot-on formulation of Indoxacarb provided >/=99.6% efficacy against flea infestations on cats for 6 weeks following a single treatment. Indoxacarb also eliminated or markedly reduced egg production for the entire evaluation period and reduced the viability of the few eggs that were produced from Day 1 through Day 38. Given Indoxacarb's effect on adult fleas, egg production and egg viability; this formulation can interrupt flea reproduction on treated cats for at least 6 weeks after treatment.


