YYA-021Competitive gp120-CD4 interaction inhibitor CAS# 144217-65-2 |
- gamma-secretase modulator 2
Catalog No.:BCC1584
CAS No.:1093978-89-2
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- Calcifediol
Catalog No.:BCC4949
CAS No.:19356-17-3
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- (24S)-24,25-Dihydroxyvitamin D3
Catalog No.:BCC1290
CAS No.:55700-58-8
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144217-65-2 | SDF | Download SDF |
| PubChem ID | 3107635 | Appearance | Powder |
| Formula | C18 H27 N3 O2 | M.Wt | 317.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (157.52 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | N'-(4-methylphenyl)-N-(2,2,6,6-tetramethylpiperidin-4-yl)oxamide | ||
| SMILES | CC1=CC=C(C=C1)NC(=O)C(=O)NC2CC(NC(C2)(C)C)(C)C | ||
| Standard InChIKey | AYAGJQPDCPRMNG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H27N3O2/c1-12-6-8-13(9-7-12)19-15(22)16(23)20-14-10-17(2,3)21-18(4,5)11-14/h6-9,14,21H,10-11H2,1-5H3,(H,19,22)(H,20,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | YYA-021 is a competitive inhibitor of the gp120-CD4 interaction. | |||||
| Targets | gp120-CD4 interaction | |||||

YYA-021 Dilution Calculator

YYA-021 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1503 mL | 15.7515 mL | 31.503 mL | 63.006 mL | 78.7575 mL |
| 5 mM | 0.6301 mL | 3.1503 mL | 6.3006 mL | 12.6012 mL | 15.7515 mL |
| 10 mM | 0.315 mL | 1.5752 mL | 3.1503 mL | 6.3006 mL | 7.8758 mL |
| 50 mM | 0.063 mL | 0.315 mL | 0.6301 mL | 1.2601 mL | 1.5752 mL |
| 100 mM | 0.0315 mL | 0.1575 mL | 0.315 mL | 0.6301 mL | 0.7876 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
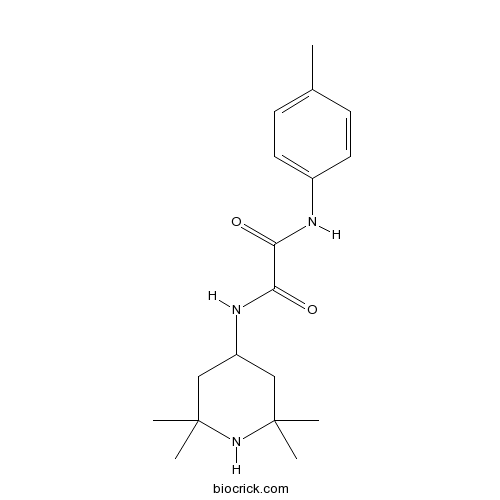
YYA-021 is a small-molecule CD4 mimic that inhibits HIV entry [1].
CD4 is a cell surface protein. The entry of human immunodeficiency virus type 1 (HIV-1) into target cells is initiated by the interaction of CD4 and viral envelope glycoprotein gp120. CD4 mimic inhibits the interaction between gp120 and CD4 and then inhibits HIV entry [1].
YYA-021 is a small-molecule CD4 mimic that inhibits HIV entry. YYA-021 competitively inhibited gp120-CD4 interaction [1]. In TZM-bl cells infected with simian-human immunodeficiency virus isolate MNA (SHIV MNA) or HIV-1 MNA pseudotyped viruses, YYA-021 inhibited the entry of Envs of SHIV MNA and HIV-1. KD-247 (the anti-V3 loop mAb, 50 μg) caused 50% neutralization of HIV-1 MNA. However, in the presence of 20 μM of YYA-021, KD-247 (<0.05 μg) caused 50% neutralization of HIV-1 MNA, which suggested that the synergistic neutralization effect of YYA-021 and KD-247. In the presence of YYA-021, SHIV MNA became more sensitive to IgG [2].
In Jcl:SD rats, the maximum tolerated dose of YYA-021.HCl was 2.5 mg. When administrated by tail vein injection, YYA-021 exhibited widespread tissue distribution due to its hydrophobicity. In a rhesus macaque, the maximum tolerated dose of YYA-021.HCl was 35.3 mg [1].
References:
[1]. Hashimoto C, Narumi T, Otsuki H, et al. A CD4 mimic as an HIV entry inhibitor: pharmacokinetics. Bioorg Med Chem, 2013, 21(24): 7884-7889.
[2]. Otsuki H, Hishiki T, Miura T, et al. Generation of a replication-competent simian-human immunodeficiency virus, the neutralization sensitivity of which can be enhanced in the presence of a small-molecule CD4 mimic. J Gen Virol, 2013, 94(Pt 12): 2710-2716.
- 1-Acetyl-4-methylpiperazine hydrochloride
Catalog No.:BCC6615
CAS No.:144205-68-5
- Indoxacarb
Catalog No.:BCN2263
CAS No.:144171-61-9
- Ledipasvir acetone
Catalog No.:BCC4046
CAS No.:1441674-54-9
- Eprosartan Mesylate
Catalog No.:BCC4658
CAS No.:144143-96-4
- Fmoc-Glu-OAll
Catalog No.:BCC3490
CAS No.:144120-54-7
- Fmoc-Asp-OAll
Catalog No.:BCC3086
CAS No.:144120-53-6
- Tarasaponin VII
Catalog No.:BCN2684
CAS No.:144118-18-3
- Deltarasin hydrochloride
Catalog No.:BCC4270
CAS No.:1440898-82-7
- Deltarasin
Catalog No.:BCC1524
CAS No.:1440898-61-2
- Febuxostat
Catalog No.:BCC2556
CAS No.:144060-53-7
- 6-O-Syringoylajugol
Catalog No.:BCN6246
CAS No.:144049-72-9
- Piclamilast
Catalog No.:BCC6215
CAS No.:144035-83-6
- Dehydroadynerigenin glucosyldigitaloside
Catalog No.:BCN1568
CAS No.:144223-70-1
- ML 337
Catalog No.:BCC6345
CAS No.:1443118-44-2
- 1-Deazaadenosine
Catalog No.:BCC6204
CAS No.:14432-09-8
- Trilepisflavan
Catalog No.:BCN6786
CAS No.:1443218-16-3
- YM 26734
Catalog No.:BCC7396
CAS No.:144337-18-8
- Nemoralisin C
Catalog No.:BCN7680
CAS No.:1443421-84-8
- CCG 203971
Catalog No.:BCC5601
CAS No.:1443437-74-8
- GSK2838232
Catalog No.:BCC6372
CAS No.:1443461-21-9
- 8-(7-Hydroxy-3,7-dimethyl-2,5-octadienyloxy)psoralen
Catalog No.:BCN1567
CAS No.:144398-34-5
- 3-Oxopomolic acid methyl ester
Catalog No.:BCN3723
CAS No.:14440-23-4
- Canophyllal
Catalog No.:BCN7441
CAS No.:14440-40-5
- 3-O-(E)-p-Coumaroylbetulin
Catalog No.:BCN6247
CAS No.:144424-80-6
A minimally cytotoxic CD4 mimic as an HIV entry inhibitor.[Pubmed:26706175]
Bioorg Med Chem Lett. 2016 Jan 15;26(2):397-400.
Several CD4 mimics have been reported as HIV-1 entry inhibitors which can block the interaction between the viral envelope glycoprotein gp120 and the cell surface protein CD4. We previously found a lead compound 2 (YYA-021) with high anti-HIV activity and low cytotoxicity. Pharmacokinetic analysis however showed compound 2 to have wide tissue distribution and relatively high distribution volumes in rats and rhesus macaques. In the present study we searched for more hydrophilic CD4 mimics with a view to reducing tissue distribution. A new compound (5) with a 1,3-benzodioxolyl moiety was found to have relatively high anti-HIV activity and no significant cytotoxicity. Compound 5 is more hydrophilic than compound 2 and the pharmacokinetics of the intravenous administration of compound 5 in a rhesus macaque showed that compound 5 has lower tissue distribution than compound 2, suggesting that compound 5 possesses a better profile.
A CD4 mimic as an HIV entry inhibitor: pharmacokinetics.[Pubmed:24189188]
Bioorg Med Chem. 2013 Dec 15;21(24):7884-9.
To date, several small molecules of CD4 mimics, which can suppress competitively the interaction between an HIV-1 envelope glycoprotein gp120 and a cellular surface protein CD4, have been reported as viral entry inhibitors. A lead compound 2 (YYA-021) with relatively high potency and low cytotoxicity has been identified previously by SAR studies. In the present study, the pharmacokinetics of the intravenous administration of compound 2 in rats and rhesus macaques is reported. The half-lives of compound 2 in blood in rats and rhesus macaques suggest that compound 2 shows wide tissue distribution and relatively high distribution volumes. A few hours after the injection, both plasma concentrations of compound 2 maintained micromolar levels, indicating it might have promise for intravenous administration when used combinatorially with anti-gp120 monoclonal antibodies.
Generation of a replication-competent simian-human immunodeficiency virus, the neutralization sensitivity of which can be enhanced in the presence of a small-molecule CD4 mimic.[Pubmed:24026672]
J Gen Virol. 2013 Dec;94(Pt 12):2710-6.
Simian-human immunodeficiency virus (SHIV) carrying the envelope from the clade B clinical human immunodeficiency virus type 1 (HIV-1) isolate MNA, designated SHIV MNA, was generated through intracellular homologous recombination. SHIV MNA inherited biological properties from the parental HIV-1, including CCR5 co-receptor preference, resistance to neutralization by the anti-V3 loop mAb KD-247 and loss of resistance in the presence of the CD4-mimic small-molecule YYA-021. SHIV MNA showed productive replication in rhesus macaque PBMCs. Experimental infection of a rhesus macaque with SHIV MNA caused a transient but high titre of plasma viral RNA and a moderate antibody response. Immunoglobulin in the plasma at 24 weeks post-infection was capable of neutralizing SHIV MNA in the presence but not in the absence of YYA-021. SHIV MNA could serve a model for development of novel therapeutic interventions based on CD4-mimic-mediated conversion of envelope protein susceptible to antibody neutralization.


