YM 26734Secretory phospholipase A2 (sPLA2) inhibitor CAS# 144337-18-8 |
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144337-18-8 | SDF | Download SDF |
| PubChem ID | 9853400 | Appearance | Powder |
| Formula | C45H62O8 | M.Wt | 730.97 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
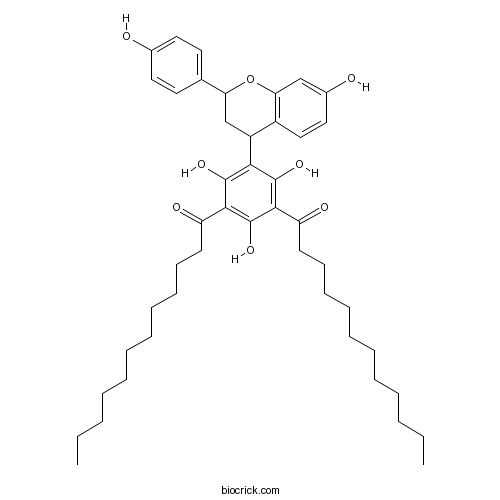
| Chemical Name | 1-[3-dodecanoyl-2,4,6-trihydroxy-5-[7-hydroxy-2-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-4-yl]phenyl]dodecan-1-one | ||
| SMILES | CCCCCCCCCCCC(=O)C1=C(C(=C(C(=C1O)C2CC(OC3=C2C=CC(=C3)O)C4=CC=C(C=C4)O)O)C(=O)CCCCCCCCCCC)O | ||
| Standard InChIKey | CEJAYJCUSZHYDS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C45H62O8/c1-3-5-7-9-11-13-15-17-19-21-36(48)41-43(50)40(44(51)42(45(41)52)37(49)22-20-18-16-14-12-10-8-6-4-2)35-30-38(31-23-25-32(46)26-24-31)53-39-29-33(47)27-28-34(35)39/h23-29,35,38,46-47,50-52H,3-22,30H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive inhibitor of secretory phospholipase A2 (sPLA2) that exhibits a broad inhibitory profile to several sPLA2s (IC50 values are 0.2, 1, 1, 1 and 3 μM for sPLA2-X, -IIA, -IID, -V and -IIE respectively). Displays minimal activity at sPLA2-IIF and no activity at cytosolic PLA2, cyclooxygenase and lipoxygenase. Ameliorates local inflammatory responses in TPA-induced mouse ear edema. |

YM 26734 Dilution Calculator

YM 26734 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.368 mL | 6.8402 mL | 13.6805 mL | 27.3609 mL | 34.2011 mL |
| 5 mM | 0.2736 mL | 1.368 mL | 2.7361 mL | 5.4722 mL | 6.8402 mL |
| 10 mM | 0.1368 mL | 0.684 mL | 1.368 mL | 2.7361 mL | 3.4201 mL |
| 50 mM | 0.0274 mL | 0.1368 mL | 0.2736 mL | 0.5472 mL | 0.684 mL |
| 100 mM | 0.0137 mL | 0.0684 mL | 0.1368 mL | 0.2736 mL | 0.342 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trilepisflavan
Catalog No.:BCN6786
CAS No.:1443218-16-3
- 1-Deazaadenosine
Catalog No.:BCC6204
CAS No.:14432-09-8
- ML 337
Catalog No.:BCC6345
CAS No.:1443118-44-2
- Dehydroadynerigenin glucosyldigitaloside
Catalog No.:BCN1568
CAS No.:144223-70-1
- YYA-021
Catalog No.:BCC5346
CAS No.:144217-65-2
- 1-Acetyl-4-methylpiperazine hydrochloride
Catalog No.:BCC6615
CAS No.:144205-68-5
- Indoxacarb
Catalog No.:BCN2263
CAS No.:144171-61-9
- Ledipasvir acetone
Catalog No.:BCC4046
CAS No.:1441674-54-9
- Eprosartan Mesylate
Catalog No.:BCC4658
CAS No.:144143-96-4
- Fmoc-Glu-OAll
Catalog No.:BCC3490
CAS No.:144120-54-7
- Fmoc-Asp-OAll
Catalog No.:BCC3086
CAS No.:144120-53-6
- Tarasaponin VII
Catalog No.:BCN2684
CAS No.:144118-18-3
- Nemoralisin C
Catalog No.:BCN7680
CAS No.:1443421-84-8
- CCG 203971
Catalog No.:BCC5601
CAS No.:1443437-74-8
- GSK2838232
Catalog No.:BCC6372
CAS No.:1443461-21-9
- 8-(7-Hydroxy-3,7-dimethyl-2,5-octadienyloxy)psoralen
Catalog No.:BCN1567
CAS No.:144398-34-5
- 3-Oxopomolic acid methyl ester
Catalog No.:BCN3723
CAS No.:14440-23-4
- Canophyllal
Catalog No.:BCN7441
CAS No.:14440-40-5
- 3-O-(E)-p-Coumaroylbetulin
Catalog No.:BCN6247
CAS No.:144424-80-6
- Aflavarin
Catalog No.:BCN7410
CAS No.:144429-67-4
- Goniodiol 8-acetate
Catalog No.:BCN4787
CAS No.:144429-71-0
- Beta-Aflatrem
Catalog No.:BCN6699
CAS No.:144446-23-1
- Clopidogrel Related Compound A
Catalog No.:BCN2687
CAS No.:144457-28-3
- Tirofiban
Catalog No.:BCC4868
CAS No.:144494-65-5
Suppression of inflammatory responses to 12-O-tetradecanoyl-phorbol-13-acetate and carrageenin by YM-26734, a selective inhibitor of extracellular group II phospholipase A2.[Pubmed:8220906]
Br J Pharmacol. 1993 Sep;110(1):447-53.
1. YM-26734 [4-(3,5-didodecanoyl-2,4,6-trihydroxyphenyl)-7-hydroxy-2-(4-hydroxyph eny l) chroman] dose-dependently inhibited the activities of extracellular phospholipase A2 (PLA2): rabbit platelet-derived group II and porcine pancreas-derived group I PLA2, with IC50 values of 0.085 (0.056-0.129, n = 5) and 6.8 (5.0-9.6, n = 5) microM, respectively. 2. In contrast, YM-26734 did not reduce the activity of intracellular PLA2 prepared from mouse macrophages, which preferentially hydrolyzed arachidonoyl phospholipids at concentrations up to 50 microM. YM-26734 also showed no effect against either sheep seminal vesicle cyclo-oxygenase or rat leukocyte 5-lipoxygenase. 3. Linewater-Burk analysis showed that YM-26567-1 behaved as a competitive inhibitor of group II PLA2 derived from rabbit platelets, with a Ki value of 48 nM. 4. In mice, YM-26734 inhibited 12-O-tetradecanoylphorbol-13-acetate (TPA, 1 microgram/ear)-induced ear oedema in a dose-dependent manner, with ED50 values of 45 (30-67) micrograms/ear (n = 5) and 11 (4-32) mg kg-1, i.v. (n = 5), but did not decrease arachidonic acid (4 mg/ear)-induced ear oedema at 1 mg/ear and 30 mg kg-1, i.v. 5. In rats, the accumulation of exudate fluids and leukocytes in the pleural cavity in response to carrageenin injection (2 mg) was significantly less in a group treated with YM-26734 (20 mg kg-1, i.v.) than in the control group (0.43 +/- 0.02 vs 0.59 +/- 0.03 g per cavity and 3.8 +/- 0.2 vs 4.9 +/- 0.3 x 10(7) cells per cavity, respectively; n = 5). 6. These results suggest that YM-26734 is a potent and competitive inhibitor of extracellular PLA2 with selectivity for group II PLA2, and that the inhibition of group II enzymes activity may cause the suppression of inflammatory responses to TPA and carrageenin.
Simplified YM-26734 inhibitors of secreted phospholipase A2 group IIA.[Pubmed:18818074]
Bioorg Med Chem Lett. 2008 Oct 15;18(20):5415-9.
Simplified analogs of YM-26734, a known inhibitor of secreted phospholipase A(2) (sPLA(2)) group IIA, were synthesized and found to also display potent inhibition at low nanomolar concentrations. Analogs were based on the didodecanoylphloroglucinol portion of YM-26734 which contains the predicted active site calcium binding group.
Induction of distinct sets of secretory phospholipase A(2) in rodents during inflammation.[Pubmed:14642775]
Biochim Biophys Acta. 2003 Nov 30;1635(1):37-47.
Although the expression of the prototypic secretory phospholipase A(2) (sPLA(2)), group IIA (sPLA(2)-IIA), is known to be up-regulated during inflammation, it remains uncertain if other sPLA(2) enzymes display similar or distinct profiles of induction under pathological conditions. In this study, we investigated the expression of several sPLA(2)s in rodent inflammation models. In lipopolysaccharide (LPS)-treated mice, the expression of sPLA(2)-V, and to a lesser extent that of sPLA(2)-IID, -IIE, and -IIF, were increased, whereas that of sPLA(2)-X was rather constant, in distinct tissues. 12-O-Tetradecanoylphorbol-13-acetate (TPA)-induced mouse ear edema, in which the expression of sPLA(2)-IID, -IIF and -V was increased, was significantly reduced by YM-26734, a competitive sPLA(2)-IIA inhibitor that turned out to inhibit sPLA(2)-IID, -IIE, -V and -X as well. In contrast, sPLA(2)-IIA was dominant in carageenin-induced pleurisy in rats, where the accumulation of exudate fluids and leukocytes was significantly ameliorated by YM-26734. These results indicate that distinct sPLA(2)s can participate in inflammatory diseases according to tissues, animal species, and types of inflammation.
Exogenous group II phospholipase A2 induces prostaglandin E2 production in mouse peritoneal macrophages.[Pubmed:8013541]
Eur J Pharmacol. 1994 Feb 21;253(1-2):155-61.
Cultures of mouse peritoneal resident macrophages produced prostaglandin E2 when exposed to extracellular group II phospholipase A2. The response to group II phospholipase A2 was concentration dependent, and prostaglandin E2 production in response to 1 microgram/ml purified group II enzyme was comparable to the maximal response elicited by lipopolysaccharide. Group II phospholipase A2 required millimolar concentrations of extracellular Ca2+ for the induction of prostaglandin E2 production, as well as for phospholipase A2 activity. YM-26734 (4-(3,5-didodecanoyl-2,4,6-trihydroxyphenyl)-7-hydroxy-2-(4-hydroxyph eny l) chroman), a selective inhibitor of group II phospholipase A2, inhibited not only the enzyme activity but also the prostaglandin E2 production-inducing activity of group II phospholipase A2 in a concentration-dependent manner. These findings suggest that group II phospholipase A2 released into the extracellular space may induce prostaglandin E2 production through hydrolysis of plasma membrane phospholipids. Taken together with the previous finding that YM-26734 suppressed inflammatory responses in vivo, these results suggest that group II phospholipase A2 may play a role in the excitation and/or progression of inflammatory processes through the production of eicosanoids.


