GSK429286ASelective ROCK1/ROCK2 inhibitor CAS# 864082-47-3 |
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- RKI-1447
Catalog No.:BCC1903
CAS No.:1342278-01-6
- Narciclasine
Catalog No.:BCN4732
CAS No.:29477-83-6
- GSK269962A
Catalog No.:BCC5178
CAS No.:850664-21-0
- SLx-2119
Catalog No.:BCC1954
CAS No.:911417-87-3
- AS 1892802
Catalog No.:BCC6335
CAS No.:928320-12-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 864082-47-3 | SDF | Download SDF |
| PubChem ID | 11373846 | Appearance | Powder |
| Formula | C21H16F4N4O2 | M.Wt | 432.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GSK429286A | ||
| Solubility | DMSO : ≥ 51 mg/mL (117.95 mM) *"≥" means soluble, but saturation unknown. | ||
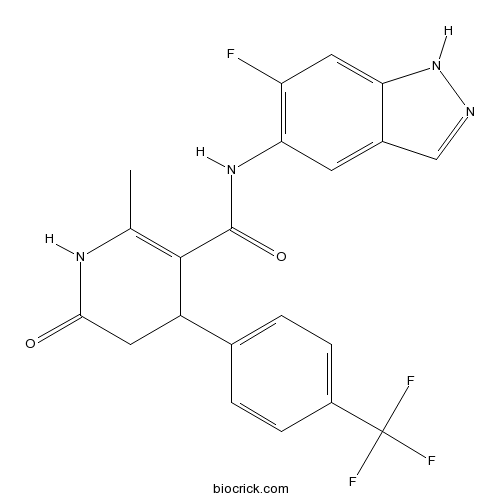
| Chemical Name | N-(6-fluoro-1H-indazol-5-yl)-6-methyl-2-oxo-4-[4-(trifluoromethyl)phenyl]-3,4-dihydro-1H-pyridine-5-carboxamide | ||
| SMILES | CC1=C(C(CC(=O)N1)C2=CC=C(C=C2)C(F)(F)F)C(=O)NC3=C(C=C4C(=C3)C=NN4)F | ||
| Standard InChIKey | OLIIUAHHAZEXEX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H16F4N4O2/c1-10-19(20(31)28-17-6-12-9-26-29-16(12)8-15(17)22)14(7-18(30)27-10)11-2-4-13(5-3-11)21(23,24)25/h2-6,8-9,14H,7H2,1H3,(H,26,29)(H,27,30)(H,28,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective Rho-kinase inhibitor (IC50 values are 14, 780 and 1940 nM for ROCK1, RSK and p70S6K respectively). Reverses adrenalin-induced contraction of the rat aortic ring (IC50 = 190 nM) and causes a dose-dependent decrease in mean arterial blood pressure in spontaneous hypertensive rats. Orally active. |

GSK429286A Dilution Calculator

GSK429286A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3128 mL | 11.5642 mL | 23.1283 mL | 46.2567 mL | 57.8208 mL |
| 5 mM | 0.4626 mL | 2.3128 mL | 4.6257 mL | 9.2513 mL | 11.5642 mL |
| 10 mM | 0.2313 mL | 1.1564 mL | 2.3128 mL | 4.6257 mL | 5.7821 mL |
| 50 mM | 0.0463 mL | 0.2313 mL | 0.4626 mL | 0.9251 mL | 1.1564 mL |
| 100 mM | 0.0231 mL | 0.1156 mL | 0.2313 mL | 0.4626 mL | 0.5782 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK429286A is a selective inhibitor of ROCK1 and ROCK2 with IC50 value of 14 nM and 63 nM, respectively [1].
Rho-kinase (ROCK) is a member of AGC (protein kinase A, protein kinase G and protein kinase C) family and plays an important role in promoting actin-myosin-mediated contractile force generation [2].
GSK429286A is a potent ROCK inhibitor and has a different activity with the reported ROCK inhibitor Y27632. Using GST method, it is shown that GSK429286A treatment (10 μM) increased MYPT phosphrylation at Thr850 via inhibiting ROCK which mediated this phosphorylation process [3].
In male Sprague-Dawley rat model with spontaneously hypertensive, oral administration of GSK429286A (30 mg/kg) marks reduced mean arterial pressure and the maximum decreased was as 50 mmHg after nearly 2 h treatment [4].
References:
[1]. Nichols, R.J., et al., Substrate specificity and inhibitors of LRRK2, a protein kinase mutated in Parkinson's disease. Biochem J, 2009. 424(1): p. 47-60.
[2]. Shi, J., et al., Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis, 2013. 4: p. e483.
[3]. Davis, D.A., et al., Increased therapeutic potential of an experimental anti-mitotic inhibitor SB715992 by genistein in PC-3 human prostate cancer cell line. BMC Cancer, 2006. 6: p. 22.
[4]. Goodman, K.B., et al., Development of dihydropyridone indazole amides as selective Rho-kinase inhibitors. J Med Chem, 2007. 50(1): p. 6-9.
- Empagliflozin (BI 10773)
Catalog No.:BCC2472
CAS No.:864070-44-0
- ZIP
Catalog No.:BCC4003
CAS No.:863987-12-6
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
- Methoxy-X04
Catalog No.:BCC6331
CAS No.:863918-78-9
- Fluconazole
Catalog No.:BCC4905
CAS No.:86386-73-4
- Methyl diacetoxy-6-gingerdiol
Catalog No.:BCN3268
CAS No.:863780-90-9
- Diacetoxy-4-gingerdiol
Catalog No.:BCN3337
CAS No.:863780-88-5
- Ganoderic acid X
Catalog No.:BCN7971
CAS No.:86377-53-9
- Ganoderic acid Y
Catalog No.:BCN2439
CAS No.:86377-52-8
- 5,8-Epidioxyergosta-6,9(11),22-trien-3-ol
Catalog No.:BCN1327
CAS No.:86363-50-0
- 6-Epiharpagide
Catalog No.:BCN4563
CAS No.:86362-16-5
- Gnetol
Catalog No.:BCN3382
CAS No.:86361-55-9
- AMG 548
Catalog No.:BCC6084
CAS No.:864249-60-5
- C 021 dihydrochloride
Catalog No.:BCC6047
CAS No.:864289-85-0
- BNTX maleate
Catalog No.:BCC6838
CAS No.:864461-31-4
- Sanggenone K
Catalog No.:BCN3373
CAS No.:86450-77-3
- Sanggenone H
Catalog No.:BCN2946
CAS No.:86450-80-8
- Nigrolineaxanthone V
Catalog No.:BCN4411
CAS No.:864516-31-4
- Sequosempervirin B
Catalog No.:BCN4777
CAS No.:864719-17-5
- Sequosempervirin D
Catalog No.:BCN4562
CAS No.:864719-19-7
- SB 699551
Catalog No.:BCC7594
CAS No.:864741-95-7
- TC-MCH 7c
Catalog No.:BCC6149
CAS No.:864756-35-4
- Gnetucleistol B
Catalog No.:BCN3585
CAS No.:864763-60-0
- Gnetucleistol C
Catalog No.:BCN3395
CAS No.:864763-61-1
Substrate specificity and inhibitors of LRRK2, a protein kinase mutated in Parkinson's disease.[Pubmed:19740074]
Biochem J. 2009 Oct 23;424(1):47-60.
The LRRK2 (leucine-rich repeat protein kinase-2) is mutated in a significant number of Parkinson's disease patients, but little is known about its regulation and function. A common mutation changing Gly2019 to serine enhances catalytic activity, suggesting that small-molecule inhibitors might have utility in treating Parkinson's disease. We employed various approaches to explore the substrate-specificity requirements of LRRK2 and elaborated a peptide substrate termed Nictide, that had 20-fold lower Km and nearly 2-fold higher Vmax than the widely deployed LRRKtide substrate. We demonstrate that LRRK2 has marked preference for phosphorylating threonine over serine. We also observed that several ROCK (Rho kinase) inhibitors such as Y-27632 and H-1152, suppressed LRRK2 with similar potency to which they inhibited ROCK2. In contrast, GSK429286A, a selective ROCK inhibitor, did not significantly inhibit LRRK2. We also identified a mutant LRRK2[A2016T] that was normally active, but resistant to H-1152 and Y-27632, as well as sunitinib, a structurally unrelated multikinase inhibitor that, in contrast with other compounds, suppresses LRRK2, but not ROCK. We have also developed the first sensitive antibody that enables measurement of endogenous LRRK2 protein levels and kinase activity as well as shRNA (short hairpin RNA) methods to reduce LRRK2 expression. Finally, we describe a pharmacological approach to validate whether substrates are phosphorylated by LRRK2 and use this to provide evidence that LRRK2 may not be rate-limiting for the phosphorylation of the proposed substrate moesin. The findings of the present study will aid with the investigation of LRRK2.
Extracellular heat shock protein 90alpha mediates HDM-induced bronchial epithelial barrier dysfunction by activating RhoA/MLC signaling.[Pubmed:28558721]
Respir Res. 2017 May 30;18(1):111.
BACKGROUND: The disruption and hyperpermeability of bronchial epithelial barrier are closely related to the pathogenesis of asthma. House dust mite (HDM), one of the most important allergens, could increase the airway epithelial permeability. Heat shock protein (Hsp) 90alpha is also implicated in the lung endothelial barrier dysfunction by disrupting RhoA signaling. However, the effect of extracellular Hsp90alpha (eHsp90alpha) on the bronchial epithelial barrier disruption induced by HDM has never been reported. METHODS: To investigate the involvement of eHsp90alpha in the bronchial epithelial barrier disruption induced by HDM, normal human bronchial epithelial cell line 16HBE14o- (16HBE) cells were treated by HDM, human recombinant (hr) Hsp90alpha and hrHsp90beta respectively and pretreated by1G6-D7, a specific anti-secreted Hsp90alpha monoclonal antibody (mAb). Hsp90alpha-silencing cells were also constructed. To further evaluate the role of RhoA signaling in this process, cells were pretreated by inhibitors of Rho kinase, GSK429286A and Y27632 2HCl. Transepithelial electrical resistance (TEER) and FITC-dextran flux (FITC-DX) were examined as the epithelial barrier function. Expression and localization of adherens junctional proteins E-cadherin and beta-catenin were evaluated by western blotting and immunofluorescence respectively. The level of eHsp90alpha was investigated by concentration and purification of condition media. RhoA activity was determined by using a Rho G-LISA(R) RhoA activation assay kit(TM) biochem kit, and the phosphorylation of myosin light chain (MLC), the downstream signal molecule of RhoA, was assessed by western blotting. RESULTS: The epithelial barrier disruption and the loss of adherens junctional proteins E-cadherin and beta-catenin in cytomembrane were observed in HDM-treated 16HBE cells, paralleled with the increase of eHsp90alpha secretion. All of which were rescued in Hsp90alpha-silencing cells or by pretreating 16HBE cells with 1G6-D7. Also, 1G6-D7 suppressed RhoA activity and MLC phosphorylation induced by HDM. Furthermore, inhibitors of Rho kinase prevented and restored the airway barrier disruption. Consistently, it was hrHsp90alpha instead of hrHsp90beta that promoted barrier dysfunction and activated RhoA/MLC signaling in 16HBE cells. CONCLUSIONS: The eHsp90alpha mediates HDM-induced human bronchial epithelial barrier dysfunction by activating RhoA/MLC signaling, suggesting that eHsp90alpha is a potential therapeutic target for treatment of asthma.
ROCK inhibition abolishes the establishment of the aquiferous system in Ephydatia muelleri (Porifera, Demospongiae).[Pubmed:26944094]
Dev Biol. 2016 Apr 15;412(2):298-310.
The Rho associated coiled-coil protein kinase (ROCK) plays crucial roles in development across bilaterian animals. The fact that the Rho/Rock pathway is required to initiate epithelial morphogenesis and thus to establish body plans in bilaterians makes this conserved signaling pathway key for studying the molecular mechanisms that may control early development of basally branching metazoans. The purpose of this study was to evaluate whether or not the main components of this signaling pathway exist in sponges, and if present, to investigate the possible role of the regulatory network in an early branching non-bilaterian species by evaluating ROCK function during Ephydatia muelleri development. Molecular phylogenetic analyses and protein domain predictions revealed the existence of Rho/Rock components in all studied poriferan lineages. Binding assays revealed that both Y-27632 and GSK429286A are capable of inhibiting Em-ROCK activity in vitro. Treatment with both drugs leads to impairment of growth and formation of the basal pinacoderm layer in the developing sponge. Furthermore, inhibition of Em-Rock prevents the establishment of a functional aquiferous system, including the absence of an osculum. In contrast, no effect of ROCK inhibition was observed in juvenile sponges that already possess a fully developed and functional aquiferous system. Thus, the Rho/Rock pathway appears to be essential for the proper development of the freshwater sponge, and may play a role in various cell behaviors (e.g. cell proliferation, cell adhesion and cell motility). Taken together, these data are consistent with an ancestral function of Rho/Rock signaling in playing roles in early developmental processes and may provide a new framework to study the interaction between Wnt signaling and the Rho/Rock pathway.
Development of dihydropyridone indazole amides as selective Rho-kinase inhibitors.[Pubmed:17201405]
J Med Chem. 2007 Jan 11;50(1):6-9.
Rho kinase (ROCK1) mediates vascular smooth muscle contraction and is a potential target for the treatment of hypertension and related disorders. Indazole amide 3 was identified as a potent and selective ROCK1 inhibitor but possessed poor oral bioavailability. Optimization of this lead resulted in the discovery of a series of dihydropyridones, exemplified by 13, with improved pharmacokinetic parameters relative to the initial lead. Indazole substitution played a critical role in decreasing clearance and improving oral bioavailability.


