C 021 dihydrochloridePotent CCR4 antagonist CAS# 864289-85-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 864289-85-0 | SDF | Download SDF |
| PubChem ID | 56972238 | Appearance | Powder |
| Formula | C27H43Cl2N5O2 | M.Wt | 540.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in water and to 100 mM in DMSO | ||
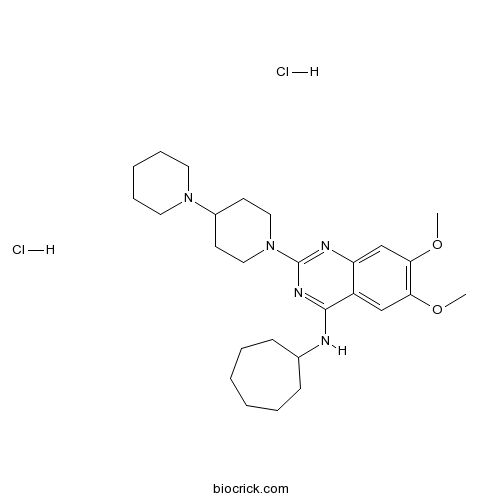
| Chemical Name | N-cycloheptyl-6,7-dimethoxy-2-(4-piperidin-1-ylpiperidin-1-yl)quinazolin-4-amine;dihydrochloride | ||
| SMILES | COC1=C(C=C2C(=C1)C(=NC(=N2)N3CCC(CC3)N4CCCCC4)NC5CCCCCC5)OC.Cl.Cl | ||
| Standard InChIKey | GKJKNYQUFAPLOQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H41N5O2.2ClH/c1-33-24-18-22-23(19-25(24)34-2)29-27(30-26(22)28-20-10-6-3-4-7-11-20)32-16-12-21(13-17-32)31-14-8-5-9-15-31;;/h18-21H,3-17H2,1-2H3,(H,28,29,30);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent CCR4 chemokine receptor antagonist (IC50 values are 0.14 and 0.039 μM for inhibition of chemotaxis in human and mouse respectively). |

C 021 dihydrochloride Dilution Calculator

C 021 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8499 mL | 9.2495 mL | 18.499 mL | 36.998 mL | 46.2475 mL |
| 5 mM | 0.37 mL | 1.8499 mL | 3.6998 mL | 7.3996 mL | 9.2495 mL |
| 10 mM | 0.185 mL | 0.9249 mL | 1.8499 mL | 3.6998 mL | 4.6247 mL |
| 50 mM | 0.037 mL | 0.185 mL | 0.37 mL | 0.74 mL | 0.9249 mL |
| 100 mM | 0.0185 mL | 0.0925 mL | 0.185 mL | 0.37 mL | 0.4625 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
C 021 dihydrochloride is a potent antagonist of CCR4 with IC50 values of 0.039 and 0.14 μM for inhibition of chemotaxis in mouse and human, respectively [1].
CC chemokine receptor 4 (CCR4) is a G protein-coupled receptor and is a receptor for the CC chemokines, which play an important role in the development and function of the immune system.
C 021 dihydrochloride is a potent CCR4 antagonist. C 021 dihydrochloride potently inhibited functional chemotaxis in mice and human with IC50 values of 0.039 and 0.14 μM, respectively. In the GTPγS-binding assay, C 021 dihydrochloride was active with IC50 value of 0.018 μM [1].
Treatment mice with azoxymethane (AOM), which induced hepatic encephalopathy, AOM significantly increased microglia activation and the concentrations of CCL2 in the liver, serum, and cortex. C 021 dihydrochloride reduced liver damage and significantly improved the neurological outcomes. Also, C 021 dihydrochloride reduced microglia activation and phosphorylation of ERK1/2, and inhibited AOM-induced cytokine upregulation [2].
References:
[1]. Yokoyama K, Ishikawa N, Igarashi S, et al. Potent and orally bioavailable CCR4 antagonists: Synthesis and structure-activity relationship study of 2-aminoquinazolines. Bioorg Med Chem, 2009, 17(1): 64-73.
[2]. McMillin M, Frampton G, Thompson M, et al. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J Neuroinflammation, 2014, 11: 121.
- AMG 548
Catalog No.:BCC6084
CAS No.:864249-60-5
- GSK429286A
Catalog No.:BCC2532
CAS No.:864082-47-3
- Empagliflozin (BI 10773)
Catalog No.:BCC2472
CAS No.:864070-44-0
- ZIP
Catalog No.:BCC4003
CAS No.:863987-12-6
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
- Methoxy-X04
Catalog No.:BCC6331
CAS No.:863918-78-9
- Fluconazole
Catalog No.:BCC4905
CAS No.:86386-73-4
- Methyl diacetoxy-6-gingerdiol
Catalog No.:BCN3268
CAS No.:863780-90-9
- Diacetoxy-4-gingerdiol
Catalog No.:BCN3337
CAS No.:863780-88-5
- Ganoderic acid X
Catalog No.:BCN7971
CAS No.:86377-53-9
- Ganoderic acid Y
Catalog No.:BCN2439
CAS No.:86377-52-8
- 5,8-Epidioxyergosta-6,9(11),22-trien-3-ol
Catalog No.:BCN1327
CAS No.:86363-50-0
- BNTX maleate
Catalog No.:BCC6838
CAS No.:864461-31-4
- Sanggenone K
Catalog No.:BCN3373
CAS No.:86450-77-3
- Sanggenone H
Catalog No.:BCN2946
CAS No.:86450-80-8
- Nigrolineaxanthone V
Catalog No.:BCN4411
CAS No.:864516-31-4
- Sequosempervirin B
Catalog No.:BCN4777
CAS No.:864719-17-5
- Sequosempervirin D
Catalog No.:BCN4562
CAS No.:864719-19-7
- SB 699551
Catalog No.:BCC7594
CAS No.:864741-95-7
- TC-MCH 7c
Catalog No.:BCC6149
CAS No.:864756-35-4
- Gnetucleistol B
Catalog No.:BCN3585
CAS No.:864763-60-0
- Gnetucleistol C
Catalog No.:BCN3395
CAS No.:864763-61-1
- Resminostat (RAS2410)
Catalog No.:BCC2165
CAS No.:864814-88-0
- Leojaponin
Catalog No.:BCN7381
CAS No.:864817-63-0
Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline.[Pubmed:25012628]
J Neuroinflammation. 2014 Jul 10;11:121.
BACKGROUND: Acute liver failure leads to systemic complications with one of the most dangerous being a decline in neurological function, termed hepatic encephalopathy. Neurological dysfunction is exacerbated by an increase of toxic metabolites in the brain that lead to neuroinflammation. Following various liver diseases, hepatic and circulating chemokines, such as chemokine ligand 2 (CCL2), are elevated, though their effects on the brain following acute liver injury and subsequent hepatic encephalopathy are unknown. CCL2 is known to activate microglia in other neuropathies, leading to a proinflammatory response. However, the effects of CCL2 on microglia activation and the pathogenesis of hepatic encephalopathy following acute liver injury remain to be determined. METHODS: Hepatic encephalopathy was induced in mice via injection of azoxymethane (AOM) in the presence or absence of INCB 3284 dimesylate (INCB), a chemokine receptor 2 inhibitor, or C 021 dihydrochloride (C021), a chemokine receptor 4 inhibitor. Mice were monitored for neurological decline and time to coma (loss of all reflexes) was recorded. Tissue was collected at coma and used for real-time PCR, immunoblots, ELISA, or immunostaining analyses to assess the activation of microglia and consequences on pro-inflammatory cytokine expression. RESULTS: Following AOM administration, microglia activation was significantly increased in AOM-treated mice compared to controls. Concentrations of CCL2 in the liver, serum, and cortex were significantly elevated in AOM-treated mice compared to controls. Systemic administration of INCB or C021 reduced liver damage as assessed by serum liver enzyme biochemistry. Administration of INCB or C021 significantly improved the neurological outcomes of AOM-treated mice, reduced microglia activation, reduced phosphorylation of ERK1/2, and alleviated AOM-induced cytokine upregulation. CONCLUSIONS: These findings suggest that CCL2 is elevated systemically following acute liver injury and that CCL2 is involved in both the microglia activation and neurological decline associated with hepatic encephalopathy. Methods used to modulate CCL2 levels and/or reduce CCR2/CCR4 activity may be potential therapeutic targets for the management of hepatic encephalopathy due to acute liver injury.
Potent and orally bioavailable CCR4 antagonists: Synthesis and structure-activity relationship study of 2-aminoquinazolines.[Pubmed:19081254]
Bioorg Med Chem. 2009 Jan 1;17(1):64-73.
Starting with a series of CC chemokine receptor-4 (CCR4) antagonists developed in a previous study, the potency was improved by replacing the pyrrolidine moiety of N-(4-chlorophenyl)-6,7-dimethoxy-2-(4-pyrrolidin-1-ylpiperidin-1-yl)quinazolin-4- amine 2 with a 3-(hydroxymethyl)piperidine. The resulting compound (1'-{4-[(4-chlorophenyl)amino]-6,7-dimethoxyquinazolin-2-yl}-1,4'-bipiperidin-3-y l)methanol 8ic was a strong inhibitor of human/mouse chemotaxis. Oral administration of 8ic showed anti-inflammatory activity in a murine model of acute dermatitis (oxazolone-induced contact hypersensitivity test) in a dose-dependent manner.


