CUDC-907Potent PI3K/HDAC inhibitor CAS# 1339928-25-4 |
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- BKM120
Catalog No.:BCC1279
CAS No.:944396-07-0
- GDC-0941
Catalog No.:BCC3626
CAS No.:957054-30-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1339928-25-4 | SDF | Download SDF |
| PubChem ID | 54575456 | Appearance | Powder |
| Formula | C23H24N8O4S | M.Wt | 508.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | fimepinostat | ||
| Solubility | DMSO : 26 mg/mL (51.13 mM; Need ultrasonic) DMF : 5 mg/mL (9.83 mM; Need ultrasonic) | ||
| Chemical Name | N-hydroxy-2-[[2-(6-methoxypyridin-3-yl)-4-morpholin-4-ylthieno[3,2-d]pyrimidin-6-yl]methyl-methylamino]pyrimidine-5-carboxamide | ||
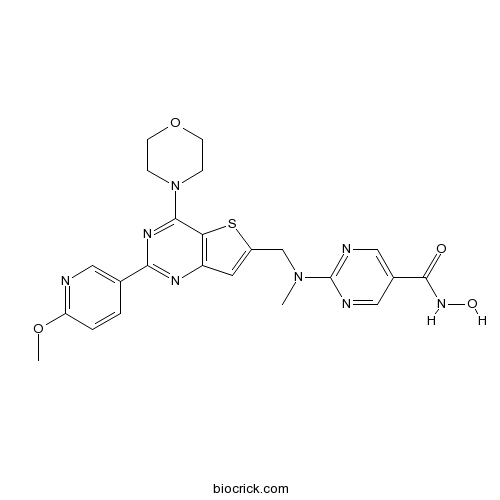
| SMILES | CN(CC1=CC2=C(S1)C(=NC(=N2)C3=CN=C(C=C3)OC)N4CCOCC4)C5=NC=C(C=N5)C(=O)NO | ||
| Standard InChIKey | JOWXJLIFIIOYMS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CUDC-907 is a dual inhibitor of PI3K and HDAC with IC50 values of 19 nM, 1.7 nM, 5 nM, 1.8 nM and 2.8 nM for PI3Kα, HDAC1, 2, 3 and 10, respectively. | ||||||
| Targets | PI3Kα | HDAC1 | HDAC2 | HDAC3 | HDAC10 | ||
| IC50 | 19 nM | 1.7 nM | 5 nM | 1.8 nM | 2.8 nM | ||

CUDC-907 Dilution Calculator

CUDC-907 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9664 mL | 9.8319 mL | 19.6637 mL | 39.3275 mL | 49.1594 mL |
| 5 mM | 0.3933 mL | 1.9664 mL | 3.9327 mL | 7.8655 mL | 9.8319 mL |
| 10 mM | 0.1966 mL | 0.9832 mL | 1.9664 mL | 3.9327 mL | 4.9159 mL |
| 50 mM | 0.0393 mL | 0.1966 mL | 0.3933 mL | 0.7865 mL | 0.9832 mL |
| 100 mM | 0.0197 mL | 0.0983 mL | 0.1966 mL | 0.3933 mL | 0.4916 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CUDC-907 is a dual-acting inhibitor of HDAC and PI3K with IC50 values of 1.7/5.0/1.8/2.8 nM (HDAC1/2/3/10) and 19/54/39 nM (PI3K).[1]
The phosphoinositide 3-kinases (PI3Ks) contain three classes of PI3K each with its own distinct lipid products and specific substrate. They are a family of lipid kinases that regulates a wide range of pathway by propagating intracellular signaling cascades. PI3K phosphorylates the 3’-OH group of phosphatidylinositols. This activated AKT, the protein Ser/Thr-kinase, by recruiting them to the cell membrane. The PI3K/AKT signaling pathway is critical in cancer, because it promotes cell growth and survival. The studies have proved that PI3K pathway plays an important role in cancer progression and treatment for lung cancers, breast cancer.[2, 3] Histone deacetylases (HDACs) and histone acetyltransferases (HATs) mediates the balance between histone deacetylation and acetylation. HDACs also regulate the acetylation status of signaling molecules, chaperones, and transcription factors that are non-histone proteins.[4]
CUDC-907 is a potent inhibitor of class I PI3K kinases and HDAC classes I and II enzymes. CUDC-907 resulted in increase of acetylated histones and non-histone proteins such as tubulin and p53. CUDC-907 also induced p21 protein in H460 cell lines. CUDC-907 dose-dependently decreases phosphorylation of AKT and its downstream targets, p70S6 and 4EBP-1 by inhibiting the PI3K pathway,, in H460 cells. CUDC-907 were able to inhibit the activation of MEK in cancer cell lines including NSCLC H460 cells, breast cancer BT-474 cells and NSCLC H1975 cells. CUDC-907 suppresses the RAF- MEK-MAPK signaling pathway through HDAC inhibition. CUDC-907 can also cause the decrease of both p-SRC) and p-SRC in RPMI-8226 multiple myeloma cells. CUDC-907 induced cell-cycle arrest at G2–M phase at the dose of 1 ?M for 24h.[1]
CUDC-907 inhibited growth of the tumor in Daudi cancer cell xenografts dose-dependently. In a xenograft tumor model of DLBCL (SU-DHL4 diffuselarge B-cell lymphoma) CUDC-907 induced tumor regression after oral administration (100 mg/kg) or intravenous (50 mg/kg). CUDC-907 also caused tumor stasis in NSCLC cell xenografts.[1]
References:
[1]. Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, Atoyan R, Qu H, Yin L, Samson M et al: Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin Cancer Res 2012, 18(15):4104-4113.
[2]. Wong KK, Engelman JA, Cantley LC: Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev 2010, 20(1):87-90.
[3]. Engelman JA: Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009, 9(8):550-562.
[4]. Kim HJ, Bae SC: Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res 2011, 3(2):166-179.
- Fmoc-Lys(2-Cl-Z)-OH
Catalog No.:BCC3513
CAS No.:133970-31-7
- Aristololactam I
Catalog No.:BCN2456
CAS No.:13395-02-3
- Rimantadine
Catalog No.:BCC4938
CAS No.:13392-28-4
- Kansuiphorin C
Catalog No.:BCN3764
CAS No.:133898-77-8
- ML224
Catalog No.:BCC5596
CAS No.:1338824-21-7
- Eicosyl ferulate
Catalog No.:BCN4712
CAS No.:133882-79-8
- Soyasaponin Ac
Catalog No.:BCN2897
CAS No.:133882-74-3
- Valnemulin HCl
Catalog No.:BCC4746
CAS No.:133868-46-9
- Safinamide
Catalog No.:BCC1915
CAS No.:133865-89-1
- OTS964
Catalog No.:BCC4025
CAS No.:1338545-07-5
- OTS514
Catalog No.:BCC4024
CAS No.:1338540-55-8
- EPZ004777
Catalog No.:BCC2218
CAS No.:1338466-77-5
- Peonidin chloride
Catalog No.:BCN3016
CAS No.:134-01-0
- Sodium ascorbate
Catalog No.:BCC4719
CAS No.:134-03-2
- Pelargonidin chloride
Catalog No.:BCN3111
CAS No.:134-04-3
- Azaguanine-8
Catalog No.:BCC4629
CAS No.:134-58-7
- (-)-Lobeline hydrochloride
Catalog No.:BCC6927
CAS No.:134-63-4
- Lobeline Sulphate
Catalog No.:BCC8203
CAS No.:134-64-5
- 4-Hydroxy-3,5-dimethoxybenzaldehyde
Catalog No.:BCN6186
CAS No.:134-96-3
- d-Laserpitin
Catalog No.:BCN3616
CAS No.:134002-17-8
- Phaseollin
Catalog No.:BCN4816
CAS No.:13401-40-6
- Methyl beta-D-fructofuranoside
Catalog No.:BCN6183
CAS No.:13403-14-0
- H-Ala-OtBu.HCl
Catalog No.:BCC3194
CAS No.:13404-22-3
- Selaginellin F
Catalog No.:BCN6420
CAS No.:1340493-24-4
CUDC-907 Promotes Bone Marrow Adipocytic Differentiation Through Inhibition of Histone Deacetylase and Regulation of Cell Cycle.[Pubmed:27829312]
Stem Cells Dev. 2017 Mar 1;26(5):353-362.
The role of bone marrow adipocytes (BMAs) in overall energy metabolism and their effects on bone mass are currently areas of intensive investigation. BMAs differentiate from bone marrow stromal cells (BMSCs); however, the molecular mechanisms regulating BMA differentiation are not fully understood. In this study, we investigated the effect of CUDC-907, identified by screening an epigenetic small-molecule library, on adipocytic differentiation of human BMSCs (hBMSCs) and determined its molecular mechanism of action. Human bone marrow stromal cells exposed to CUDC-907 (500 nM) exhibited enhanced adipocytic differentiation ( approximately 2.9-fold increase, P < 0.005) compared with that of control cells. Global gene expression and signaling pathway analyses of differentially expressed genes revealed a strong enrichment of genes involved in adipogenesis, cell cycle, and DNA replication. Chromatin immune precipitation combined with quantitative polymerase chain reaction showed significant increase in H3K9ac epigenetic marker in the promoter regions of AdipoQ, FABP4, PPARgamma, KLF15, and CEBPA in CUDC-907-treated hBMSCs. Follow-up experiments corroborated that the inhibition of histone deacetylase (HDAC) activity enhanced adipocytic differentiation, while the inhibition of PI3K decreased adipocytic differentiation. In addition, CUDC-907 arrested hBMSCs in the G0-G1 phase of the cell cycle and reduced the number of S-phase cells. Our data reveal that HDAC, PI3K, and cell cycle genes are important regulators of BMA formation and demonstrate that adipocyte differentiation of hBMSCs is associated with complex changes in a number of epigenetic and genetic pathways, which can be targeted to regulate BMA formation.
Safety, tolerability, and preliminary activity of CUDC-907, a first-in-class, oral, dual inhibitor of HDAC and PI3K, in patients with relapsed or refractory lymphoma or multiple myeloma: an open-label, dose-escalation, phase 1 trial.[Pubmed:27049457]
Lancet Oncol. 2016 May;17(5):622-31.
BACKGROUND: Treatment options for patients with relapsed or refractory lymphoma and multiple myeloma are limited. CUDC-907 is an oral, first-in-class, small molecule that is designed to inhibit both histone deacetylase (HDAC) and PI3K enzymes, which are members of common oncogenic pathways in haematological malignancies. We aimed to assess overall safety and preliminary activity in this dose-escalation study of CUDC-907 monotherapy in patients with relapsed or refractory lymphoma and multiple myeloma. METHODS: This open-label, first-in-man, phase 1 trial recruited adult patients (aged >/=18 years) with lymphoma or multiple myeloma who were refractory to or had relapsed after two or more previous regimens, from four US cancer centres. CUDC-907 was orally administered in a standard 3 + 3 dose-escalation design at four different dosing schedules, to which participants were sequentially assigned as follows: once daily, intermittently (twice or three times weekly; simultaneous enrolment), and daily for 5 days followed by a 2-day break (5/2), in 21-day cycles. Dosing started at 30 mg for the once-daily schedule and 60 mg for other schedules, escalating in 30 mg increments. Patients continued to receive CUDC-907 until disease progression or until other treatment discontinuation criteria were met. The primary objective was to determine the maximum tolerated dose (MTD) and recommended phase 2 dose, assessed in patients who received at least 66% of cycle 1 doses without modification and those who had a dose-limiting toxicity (DLT) in cycle 1 irrespective of dose modification. We assessed safety in all patients who received at least one dose of study drug. This ongoing trial is registered at ClinicalTrials.gov, number NCT01742988. FINDINGS: Between Jan 23, 2013, and July 27, 2015, we enrolled 44 patients, of whom ten were sequentially assigned to CUDC-907 once-daily (MTD 60 mg), 12 to twice-weekly (MTD 150 mg), 15 to three-times-weekly (MTD 150 mg), and seven to the 5/2 dosing schedule (MTD 60 mg). 37 (84%) patients had discontinued study drug as a result of progressive disease or clinical signs of progressive disease at the data cutoff. Four DLTs occurred in three of 40 DLT-evaluable patients (diarrhoea and hyperglycaemia in one patient on 60 mg once daily, hyperglycaemia in one patient on 150 mg twice weekly, and diarrhoea in one patient on 150 mg three times weekly); no DLTs were reported in patients on the 5/2 schedule. Grade 3 or worse adverse events occurred in 19 (43%) of 44 patients, the most common of which were thrombocytopenia (in nine [20%] of 44 patients), neutropenia (three [7%]), and hyperglycaemia (three [7%]). 11 (25%) of 44 patients had serious adverse events, three of which were regarded as treatment related (epistaxis and the DLTs of diarrhoea and hyperglycaemia). Adverse events led to dose reductions in six (14%) patients and treatment discontinuation in seven (16%). Five (14%) of 37 response-evaluable patients achieved an objective response (two complete responses and three partial responses). All five responses occurred in the subgroup of patients with diffuse large B-cell lymphoma (DLBCL; n=9), and three occurred in those with transformed follicular lymphoma DLBCL (n=5). 21 (57%) of 37 response-evaluable patients had stable disease, including those with DLBCL, Hodgkin's lymphoma, and multiple myeloma. On the basis of these findings, we selected CUDC-907 60 mg on the 5/2 dosing schedule as the recommended phase 2 dose. INTERPRETATION: The safety and tolerability profile of CUDC-907 and the promising preliminary evidence of response support continued development of CUDC-907 at the 60 mg 5/2 dosing schedule, alone and in combination with other therapies. A dose-expansion trial of this dose in patients with refractory and relapsed DLBCL in particular, is ongoing. FUNDING: Curis, Inc, and the Leukemia and Lymphoma Society.
Dual HDAC and PI3K Inhibitor CUDC-907 Downregulates MYC and Suppresses Growth of MYC-dependent Cancers.[Pubmed:27980108]
Mol Cancer Ther. 2017 Feb;16(2):285-299.
Upregulation of MYC is a common driver event in human cancers, and some tumors depend on MYC to maintain transcriptional programs that promote cell growth and proliferation. Preclinical studies have suggested that individually targeting upstream regulators of MYC, such as histone deacetylases (HDAC) and phosphoinositide 3-kinases (PI3K), can reduce MYC protein levels and suppress the growth of MYC-driven cancers. Synergy between HDAC and PI3K inhibition in inducing cancer cell death has also been reported, but the involvement of MYC regulation is unclear. In this study, we demonstrated that HDAC and PI3K inhibition synergistically downregulates MYC protein levels and induces apoptosis in "double-hit" (DH) diffuse large B-cell lymphoma (DLBCL) cells. Furthermore, CUDC-907, a small-molecule dual-acting inhibitor of both class I and II HDACs and class I PI3Ks, effectively suppresses the growth and survival of MYC-altered or MYC-dependent cancer cells, such as DH DLBCL and BRD-NUT fusion-positive NUT midline carcinoma (NMC) cells, and MYC protein downregulation is an early event induced by CUDC-907 treatment. Consistently, the antitumor activity of CUDC-907 against multiple MYC-driven cancer types was also demonstrated in animal models, including DLBCL and NMC xenograft models, Myc transgenic tumor syngeneic models, and MYC-amplified solid tumor patient-derived xenograft (PDX) models. Our findings suggest that dual function HDAC and PI3K inhibitor CUDC-907 is an effective agent targeting MYC and thus may be developed as potential therapy for MYC-dependent cancers. Mol Cancer Ther; 16(2); 285-99. (c)2016 AACR.
Enhanced efficacy of 5-fluorouracil in combination with a dual histone deacetylase and phosphatidylinositide 3-kinase inhibitor (CUDC-907) in colorectal cancer cells.[Pubmed:28139498]
Saudi J Gastroenterol. 2017 Jan-Feb;23(1):34-38.
BACKGROUND/AIMS: 5-Fluorouracil (5-FU) is widely used in the treatment of patients with colorectal cancer (CRC). However, the efficacy of 5-FU as a single agent is limited, with multiple undesired side effects. Therefore, the aim of the current study was to assess the efficacy of CUDC-907 (a dual inhibitor of histone deacetylase and phosphatidylinositide 3-kinase) in combination with 5-FU against CRC cells. MATERIALS AND METHODS: Cell viability was determined using AlamarBlue and colony formation assays. Acridine orange/ethidium bromide staining and flow cytometry were used to measure apoptotic and necrotic events, as well as cell cycle progression. Immunoblotting was used to assess acetylation of histone H3 and phosphorylation of AKT. RESULTS: Our data revealed enhanced toxicity of CUDC-907 against HCT116, RKO, COLO-205, and HT-29 CRC cells when combined with 5-FU. Similarly, the colony formation capability of HCT116 cells was suppressed by the combination treatment. Cells treated with CUDC-907 and 5-FU underwent apoptosis and necrosis, and exhibited increased polyploidy. Furthermore, CRC cells treated with CUDC-907 exhibited a higher degree of histone H3 lysine 9 acetylation (H3K9ac) and reduced AKT phosphorylation (Ser473). CONCLUSION: Our data revealed, for the first time, the enhanced inhibitory effect of CUDC-907 against CRC cells when combined with 5-FU, supporting the application of this combination as a potential therapeutic strategy in CRC treatment.


