Panobinostat (LBH589)HDAC inhibitor CAS# 404950-80-7 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 404950-80-7 | SDF | Download SDF |
| PubChem ID | 6918837 | Appearance | Powder |
| Formula | C21H23N3O2 | M.Wt | 349.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LBH589; NVP-LBH589 | ||
| Solubility | DMSO : ≥ 57 mg/mL (163.12 mM) *"≥" means soluble, but saturation unknown. | ||
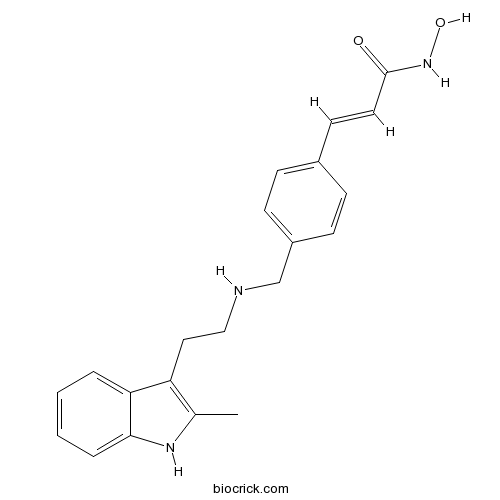
| Chemical Name | (E)-N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]prop-2-enamide | ||
| SMILES | CC1=C(C2=CC=CC=C2N1)CCNCC3=CC=C(C=C3)C=CC(=O)NO | ||
| Standard InChIKey | FPOHNWQLNRZRFC-ZHACJKMWSA-N | ||
| Standard InChI | InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Panobinostat (LBH589) is a novel broad-spectrum inhibitor of HDAC with IC50 of 5 nM. | |||||
| Targets | HDAC (MOLT-4 cells) | HDAC (Reh cells) | ||||
| IC50 | 5 nM | 20 nM | ||||
| Cell experiment [1]: | |
| Cell lines | MCF-7aro, LTEDaro, Exe-R, Let-R, Ana-R cell lins |
| Preparation method | The solubility of this compound in DMSO is <10 mm. general tips for obtaining a higher concentration: please warm the tube at 37 °c 10 minutes and> |
| Reacting condition | 6d; 20 nM |
| Applications | To study cellular response to AIs and the mechanisms of acquired AI resistance, we used the previously generated AI-responsive cell line MCF-7aro and AI-resistant variants of MCF-7aro created following in vitro selection against each AI (i.e., Exe-R, Let-R, and Ana-R) or long-term culture in the absence of estrogen (i.e., LTEDaro). MCF-7aro, LTEDaro and three AI-resistant cell lines were exposed to increasing concentrations of LBH589. This drug-inhibited proliferation of all cell lines in a dose-dependent manner. |
| Animal experiment [1]: | |
| Animal models | Female, 6- to 7-week-old ovariectomized, BALB/c Nu–Nu athymic mice |
| Dosage form | 20 mg/kg, three times per week, intraperitoneal injection |
| Application | To evaluate the inhibitory effects of LBH589 on AI resistance in vivo, we used the exemestane-resistant MCF7aro xenograft model. LBH589 treatment significantly inhibited the growth of exemestane-resistant tumors; tumor weight at the end of experiment was significantly lesser in mice treated with LBH589 than in control mice. No mice in the LBH589 treat-ment groups showed significant body weight loss indicating that the LBH589 treatment was well tolerated. Consistent with the effect of LBH589 on gross character-istics of the tumors, proliferation (assessed by Ki-67 staining) of tumor cells was significantly decreased in LBH589-treated mice and apoptosis (assessed by staining for cleaved PARP) of tumor cells was significantly increased. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Kubo M, Kanaya N, Petrossian K, et al. Inhibition of the proliferation of acquired aromatase inhibitor-resistant breast cancer cells by histone deacetylase inhibitor LBH589 (panobinostat)[J]. Breast cancer research and treatment, 2013, 137(1): 93-107. | |

Panobinostat (LBH589) Dilution Calculator

Panobinostat (LBH589) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8618 mL | 14.309 mL | 28.618 mL | 57.2361 mL | 71.5451 mL |
| 5 mM | 0.5724 mL | 2.8618 mL | 5.7236 mL | 11.4472 mL | 14.309 mL |
| 10 mM | 0.2862 mL | 1.4309 mL | 2.8618 mL | 5.7236 mL | 7.1545 mL |
| 50 mM | 0.0572 mL | 0.2862 mL | 0.5724 mL | 1.1447 mL | 1.4309 mL |
| 100 mM | 0.0286 mL | 0.1431 mL | 0.2862 mL | 0.5724 mL | 0.7155 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Panobinostat, as known as LBH589, is a novel and potent hydroxamic acid-based deacetylase inhibitor (DACis)that inhibits a broad spectrum of histone deacetylases (HDACs), including all Classes 1, 2 and 4 HDAC enzymes, at low nanomolar concentrations. According to previous studies, it not only induces apoptosis in multiple myeloma (MM) cells via caspase activation and poly(ADP-ribose) polymerase (PARP) cleavage, but also induces potent cell growth inhibition, cell-cycle arrest, and apoptosis in a time- and dose-dependent manner in both Philadelphia chromosome-negative (Ph-) actue lymphoblastic leukemia (ALL) cells lines (T-cell MOLT-4 and pre-B-cell Reh), which are correlated with induction of histone (H3K9 and H4K8) hyperacetylation, activation of p21 and p27, and suppression of c-Myc.
Reference
Wenlin Shao, Joseph D. Growney, Yun Feng, Gregory O’Connor, Minying Pu, Wenjing Zhu, Yung-Mae Yao, Paul Kwon, Stephen Fawell and Peter Atadja. Activity of deacetylase inhibitor panobinostat (LBH589) in cutaneous T-cell lymphoma models: defining molecular mechanisms of resistance. Int. J. Cancer: 127, 2199-2208 (2010)
Laurence Catley, Ellen Weisberg, Tanyel Kiziltepe, Yu-Tzu Tai, Teru Hideshima, Paola Neri, Pierfrancesco Tassone, Peter Atadja, Dharminder Chauhan, Nikhil C. Munshi and Keneth C. Anderson. Aggresome induction by proteasome inhibitor bortezomib and α-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood (2006); 108(10): 3441-3449
Anna Scuto, Mark Kirschbaum, Claudia Kowolik, Leo Kretzner, Agnes Juhasz, Peter Atadja, Vinod Pullarkat, Ravi Bhatia, Stephen Forman, Yun Yen, and Richard Jove. The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells. Blood (2008); 111(10):5093-5100
- Ethyl ferulate
Catalog No.:BCN1257
CAS No.:4046-02-0
- Bursehernin
Catalog No.:BCN3040
CAS No.:40456-51-7
- Yatein
Catalog No.:BCN5456
CAS No.:40456-50-6
- Kaempferol-3-O-glucorhamnoside
Catalog No.:BCN2830
CAS No.:40437-72-7
- Pinusolidic acid
Catalog No.:BCN5455
CAS No.:40433-82-7
- erythro-1-Phenylpropane-1,2-diol
Catalog No.:BCN6596
CAS No.:40421-52-1
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Pamidronate
Catalog No.:BCC4693
CAS No.:40391-99-9
- 6-Methoxykaempferol 3-O-rutinoside
Catalog No.:BCN2379
CAS No.:403861-33-6
- Benzyl 2-hydroxy-6-(beta-glucosyloxy)benzoate
Catalog No.:BCN7434
CAS No.:403857-21-6
- HEMADO
Catalog No.:BCC7118
CAS No.:403842-38-6
- 10058-F4
Catalog No.:BCC1050
CAS No.:403811-55-2
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
- Z-Lys(Z)-OH
Catalog No.:BCC2762
CAS No.:405-39-0
- 4'-Demethylpodophyllotoxin
Catalog No.:BCN2625
CAS No.:40505-27-9
- Salubrinal
Catalog No.:BCC4843
CAS No.:405060-95-9
- Drechslerine A
Catalog No.:BCN7561
CAS No.:405157-84-8
- Drechslerine D
Catalog No.:BCN7502
CAS No.:405157-88-2
- Besifloxacin HCl
Catalog No.:BCC4764
CAS No.:405165-61-9
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- Dovitinib (TKI-258, CHIR-258)
Catalog No.:BCC1169
CAS No.:405169-16-6
- NFPS
Catalog No.:BCC7484
CAS No.:405225-21-0
- Dadahol A
Catalog No.:BCN5457
CAS No.:405281-76-7
- Cyclapolin 9
Catalog No.:BCC7571
CAS No.:40533-25-3
Influence of a novel histone deacetylase inhibitor panobinostat (LBH589) on the growth of ovarian cancer.[Pubmed:27633667]
J Ovarian Res. 2016 Sep 15;9(1):58.
BACKGROUND: Pre-clinical studies have demonstrated that natural and synthetic histone deacetylase (HDAC) inhibitors can impede the in vitro and in vivo growth of cell lines from a variety of gynecologic and other malignancies. We investigated the anti-tumor activity of Panobinostat (LBH589) both in vitro and in vivo as either a single agent or in combination with conventional cytotoxic chemotherapy using patient-derived xenograft (PDX) models of primary serous ovarian tumors. METHODS: The ovarian cancer cell lines OVCAR8, SKOV3 and their paclitaxel-resistant derivatives OVCAR8-TR and SKOV3-TR were treated with increasing doses of LBH589. The effect of LBH589 on cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Serially transplanted primary human high-grade serous ovarian adenocarcinoma tissue was utilized to generate xenografts in 6-week old female NOD/SCID mice. The mice were then randomized into one of 4 treatment groups: (1) vehicle control; (2) paclitaxel and carboplatin (P/C); (3) LBH589; or (4) P/C + LBH589. Mice were treated for 21 days and tumor volumes and mouse weights were obtained every 3 days. These experiments were performed in triplicate with three different patient derived tumors. Wilcoxan rank-sum testing was utilized to assess tumor volume differences. RESULTS: In vitro treatment with LBH589 significantly reduced the viability of both taxol-sensitive and taxol-resistant ovarian cancer cell lines (p < 0.01). In vivo treatment with LBH589 alone appeared tumorstatic and reduced tumor growth when compared to vehicle treatment (p < 0.007) after 21 days. This single agent activity was confirmed in two additional experiments with other PDX tumors (p < 0.03, p < 0.05). A potential additive effect of LBH589 and P/C, manifested as enhanced tumor regression with the addition of LBH589 compared to vehicle (p < 0.02), in one of the three analyzed serous PDX models. CONCLUSIONS: Our findings suggest that pan-HDAC inhibition with panobinostat precludes the growth of ovarian cancer cell lines in vitro and PDXs in vivo. Added benefit of LBH589 to standard P/C therapy was observed in one of three PDX models suggesting improved response in a subset of serous ovarian cancers.
Phase I Study of Panobinostat (LBH589) and Letrozole in Postmenopausal Metastatic Breast Cancer Patients.[Pubmed:26774555]
Clin Breast Cancer. 2016 Apr;16(2):82-6.
INTRODUCTION: Histone deacetylase inhibitors have been found to restore sensitivity to the estrogen receptor in endocrine-resistant and triple-negative breast cancer cell lines. We decided to test panobinostat, a pan-histone deacetylase inhibitor, because of preclinical data, combined with letrozole in a phase I study. PATIENTS AND METHODS: We enrolled patients with metastatic breast cancer to determine the safety and tumor response using Response Evaluation Criteria In Solid Tumors. Dose level 1 was panobinostat 20 mg orally 3 times weekly with oral letrozole 2.5 mg daily. Dose level 2 was panobinostat 30 mg orally 3 times weekly, with the same dose of letrozole. RESULTS: A total of 12 patients (6 at each dose level) were enrolled, and 43 cycles of treatment were given. Of the 6 patients at dose level 1, 1 experienced dose-limiting toxicity (20-mg dose level; an increase in creatinine). At the 30-mg dose level, 3 of 6 patients experienced dose-limiting toxicity, 1 each of grade 3 thrombocytopenia with bleeding, grade 4 thrombocytopenia, and grade 3 diarrhea. The maximum tolerated dose was 20 mg. Of the 12 patients, 2 experienced a partial response, and 5 had stable disease. The most common severe adverse event was thrombocytopenia, occurring in 4 of 12 patients. CONCLUSION: The recommended phase II starting dose is panobinostat 20 mg orally 3 times weekly (eg, Monday, Wednesday, Friday) and oral letrozole 2.5 mg daily. This dose should be escalated to 30 mg orally 3 times weekly if no grade 3 toxicity has developed, because the partial responses occurred in patients receiving the 30-mg dose.
Convection enhanced delivery of panobinostat (LBH589)-loaded pluronic nano-micelles prolongs survival in the F98 rat glioma model.[Pubmed:28260886]
Int J Nanomedicine. 2017 Feb 21;12:1385-1399.
BACKGROUND: The pan-histone deacetylase inhibitor panobinostat is a potential therapy for malignant glioma, but it is water insoluble and does not cross the blood-brain barrier when administered systemically. In this article, we describe the in vitro and in vivo efficacy of a novel water-soluble nano-micellar formulation of panobinostat designed for administration by convection enhanced delivery (CED). MATERIALS AND METHODS: The in vitro efficacy of panobinostat-loaded nano-micelles against rat F98, human U87-MG and M059K glioma cells and against patient-derived glioma stem cells was measured using a cell viability assay. Nano-micelle distribution in rat brain was analyzed following acute CED using rhodamine-labeled nano-micelles, and toxicity was assayed using immunofluorescent microscopy and synaptophysin enzyme-linked immunosorbent assay. We compared the survival of the bioluminescent syngenic F98/Fischer344 rat glioblastoma model treated by acute CED of panobinostat-loaded nano-micelles with that of untreated and vehicle-only-treated controls. RESULTS: Nano-micellar panobinostat is cytotoxic to rat and human glioma cells in vitro in a dose-dependent manner following short-time exposure to drug. Fluorescent rhodamine-labelled nano-micelles distribute with a volume of infusion/volume of distribution (Vi/Vd) ratio of four and five respectively after administration by CED. Administration was not associated with any toxicity when compared to controls. CED of panobinostat-loaded nano-micelles was associated with significantly improved survival when compared to controls (n=8 per group; log-rank test, P<0.001). One hundred percent of treated animals survived the 60-day experimental period and had tumour response on post-mortem histological examination. CONCLUSION: CED of nano-micellar panobinostat represents a potential novel therapeutic option for malignant glioma and warrants translation into the clinic.
A phase I trial of panobinostat (LBH589) in patients with metastatic melanoma.[Pubmed:27748045]
Cancer Med. 2016 Nov;5(11):3041-3050.
Epigenetic alterations by histone/protein deacetylases (HDACs) are one of the many mechanisms that cancer cells use to alter gene expression and promote growth. HDAC inhibitors have proven to be effective in the treatment of specific malignancies, particularly in combination with other anticancer agents. We conducted a phase I trial of panobinostat in patients with unresectable stage III or IV melanoma. Patients were treated with oral panobinostat at a dose of 30 mg daily on Mondays, Wednesdays, and Fridays (Arm A). Three of the six patients on this dose experienced clinically significant thrombocytopenia requiring dose interruption. Due to this, a second treatment arm was opened and the dose was changed to 30 mg oral panobinostat three times a week every other week (Arm B). Six patients were treated on Arm A and 10 patients were enrolled to Arm B with nine patients treated. In nine patients treated on Arm B, the response rate was 0% (90% confidence interval [CI]: 0-28%) and the disease-control rate was 22% (90% CI: 4-55%). Among all 15 patients treated, the overall response rate was 0% (90% CI: 0-17%) and the disease-control rate was 27% (90% CI: 10-51%). There was a high rate of toxicity associated with treatment. Correlative studies suggest the presence of immune modifications after HDAC inhibition. Panobinostat is not active as a single agent in the treatment of melanoma. Further exploration of this agent in combination with other therapies may be warranted.


