YateinCAS# 40456-50-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40456-50-6 | SDF | Download SDF |
| PubChem ID | 442835 | Appearance | Powder |
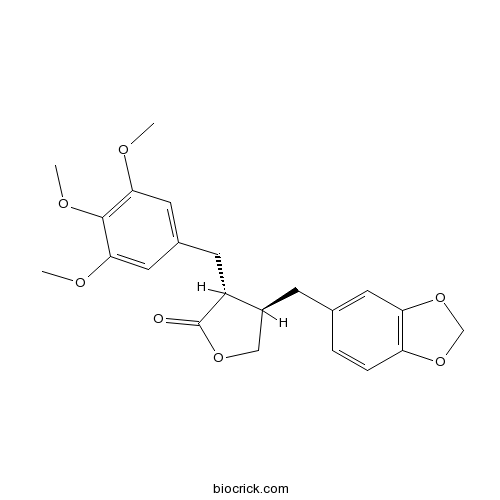
| Formula | C22H24O7 | M.Wt | 400.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | (-)-Deoxypodorhizone; Dihydroanhydropodorhizol | ||
| Solubility | Soluble in chloroform; insoluble in water | ||
| Chemical Name | (3R,4R)-4-(1,3-benzodioxol-5-ylmethyl)-3-[(3,4,5-trimethoxyphenyl)methyl]oxolan-2-one | ||
| SMILES | COC1=CC(=CC(=C1OC)OC)CC2C(COC2=O)CC3=CC4=C(C=C3)OCO4 | ||
| Standard InChIKey | GMLDZDDTZKXJLU-JKSUJKDBSA-N | ||
| Standard InChI | InChI=1S/C22H24O7/c1-24-19-9-14(10-20(25-2)21(19)26-3)7-16-15(11-27-22(16)23)6-13-4-5-17-18(8-13)29-12-28-17/h4-5,8-10,15-16H,6-7,11-12H2,1-3H3/t15-,16+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Yatein is a lignan precursor of podophyllotoxin, a key agent in anticancer drugs. 2. Yatein can significantly suppress HSV-1 multiplication in HeLa cells without apparent cytotoxicity. |
| Targets | DNA/RNA Synthesis | HSV |

Yatein Dilution Calculator

Yatein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4975 mL | 12.4875 mL | 24.975 mL | 49.95 mL | 62.4376 mL |
| 5 mM | 0.4995 mL | 2.4975 mL | 4.995 mL | 9.99 mL | 12.4875 mL |
| 10 mM | 0.2498 mL | 1.2488 mL | 2.4975 mL | 4.995 mL | 6.2438 mL |
| 50 mM | 0.05 mL | 0.2498 mL | 0.4995 mL | 0.999 mL | 1.2488 mL |
| 100 mM | 0.025 mL | 0.1249 mL | 0.2498 mL | 0.4995 mL | 0.6244 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kaempferol-3-O-glucorhamnoside
Catalog No.:BCN2830
CAS No.:40437-72-7
- Pinusolidic acid
Catalog No.:BCN5455
CAS No.:40433-82-7
- erythro-1-Phenylpropane-1,2-diol
Catalog No.:BCN6596
CAS No.:40421-52-1
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Pamidronate
Catalog No.:BCC4693
CAS No.:40391-99-9
- 6-Methoxykaempferol 3-O-rutinoside
Catalog No.:BCN2379
CAS No.:403861-33-6
- Benzyl 2-hydroxy-6-(beta-glucosyloxy)benzoate
Catalog No.:BCN7434
CAS No.:403857-21-6
- HEMADO
Catalog No.:BCC7118
CAS No.:403842-38-6
- 10058-F4
Catalog No.:BCC1050
CAS No.:403811-55-2
- Qc 1
Catalog No.:BCC6356
CAS No.:403718-45-6
- Cannabisin H
Catalog No.:BCN3896
CAS No.:403647-08-5
- Obtusafuran methyl ether
Catalog No.:BCN8103
CAS No.:40357-59-3
- Bursehernin
Catalog No.:BCN3040
CAS No.:40456-51-7
- Ethyl ferulate
Catalog No.:BCN1257
CAS No.:4046-02-0
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
- Z-Lys(Z)-OH
Catalog No.:BCC2762
CAS No.:405-39-0
- 4'-Demethylpodophyllotoxin
Catalog No.:BCN2625
CAS No.:40505-27-9
- Salubrinal
Catalog No.:BCC4843
CAS No.:405060-95-9
- Drechslerine A
Catalog No.:BCN7561
CAS No.:405157-84-8
- Drechslerine D
Catalog No.:BCN7502
CAS No.:405157-88-2
- Besifloxacin HCl
Catalog No.:BCC4764
CAS No.:405165-61-9
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- Dovitinib (TKI-258, CHIR-258)
Catalog No.:BCC1169
CAS No.:405169-16-6
Yatein from Chamaecyparis obtusa suppresses herpes simplex virus type 1 replication in HeLa cells by interruption the immediate-early gene expression.[Pubmed:16540181]
Antiviral Res. 2006 Jul;70(3):112-20.
Inhibitory effects of methanolic extracts from nine Chinese herbs on herpes simplex virus type 1 (HSV-1) replication were studied. By a bioassay-guided fractionation procedure, Yatein (C(22)H(23)O(7); M.W.399) was isolated from Chamaecyparis obtusa; Yatein significantly suppressed HSV-1 multiplication in HeLa cells without apparent cytotoxicity. To further localize the point in the HSV-1 replication cycle where arrest occurred, a set of key regulatory events leading to the viral multiplication was examined, including viral immediate-early (alpha) and late (gamma) gene expression and DNA replication. Results indicated that levels of glycoprotein B (gB) and gC mRNA expression in HeLa cells were impeded by Yatein. Data from polymerase chain reaction showed that replication of HSV-1 DNA in HeLa cells was arrested by Yatein. Furthermore, Yatein decreased ICP0 and ICP4 gene expression in HeLa cells. Results of an electrophoretic mobility shift assay demonstrated that Yatein interrupted the formation of alpha-trans-induction factor/C1/Oct-1/GARAT multiprotein complex. The mechanisms of antiviral action of Yatein seem to be mediated, by inhibiting HSV-1 alpha gene expression, including expression of the ICP0 and ICP4 genes, and by arresting HSV-1 DNA synthesis and structural protein expression in HeLa cells. These results suggest that Yatein is an antiviral agent against HSV-1 replication.
Antiproliferative activity of yatein isolated from Austrocedrus chilensis against murine myeloma cells: cytological studies and chemical investigations.[Pubmed:25420758]
Pharm Biol. 2015 Mar;53(3):378-85.
CONTEXT: Fitzroya cupressoides (Molina) I. M. Johnst. and Austrocedrus chilensis (D. Don) Pic.Serm. & Bizzarri are two Chilean Cupressaceae that are naturally resistant to biodegradation. Secondary metabolites from these species display a variety of biological activities. OBJECTIVE: To evaluate the antiproliferative activity of two lignans, a diterpene and a flavonol isolated from A. chilensis and F. cupressoides, to elucidate their cytological effects on P3X murine myeloma cells. MATERIALS AND METHODS: The antiproliferative activity of Yatein, isotaxiresinol, ferruginol, and isorhamnetin was evaluated in vitro using the MTT assay. The effect of Yatein at the cellular level, due to its high antiproliferative activity was evaluated. P3X cells treated for 24 h with 12.5 and 25 microg/mL of Yatein were also examined at the cytological level using immunofluorescence and scanning and transmission electron microscopy. RESULTS: Yatein, a lignan isolated from A. chilensis, potentially inhibited P3X murine myeloma cell proliferation, resulting in approximately 75% cell death in response to a 25 microg/mL treatment with the lignan. P3X cells lost membrane integrity at the nuclear and cytoplasmic levels, including organelles, in response to Yatein treatment (12.5 microg/mL), and we observed changes in the cytoplasmic organization and distribution of microtubules. The other compounds tested had low activity. DISCUSSION AND CONCLUSIONS: Yatein is a lignan precursor of podophyllotoxin, a key agent in anticancer drugs. Due to its structural similarities to podophyllotoxin, Yatein could have similar cytoplasmic target(s), such as the microtubular apparatus. These findings suggest that Yatein may be of potential pharmacological interest and warrants further investigation in human cell lines.


