CapsaicinTRPV1 receptor agonist CAS# 404-86-4 |
- Baicalein
Catalog No.:BCN5599
CAS No.:491-67-8
- Luteolin
Catalog No.:BCN5600
CAS No.:491-70-3
- Chloroquine diphosphate
Catalog No.:BCC3915
CAS No.:50-63-5
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- Vitamin D3
Catalog No.:BCN2186
CAS No.:67-97-0
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 404-86-4 | SDF | Download SDF |
| PubChem ID | 1548943 | Appearance | White powder |
| Formula | C18H27NO3 | M.Wt | 305.42 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | (E)-Capsaicin; 8-Methyl-N-vanillyl-trans-6-nonenamide | ||
| Solubility | DMSO : ≥ 44 mg/mL (144.07 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide | ||
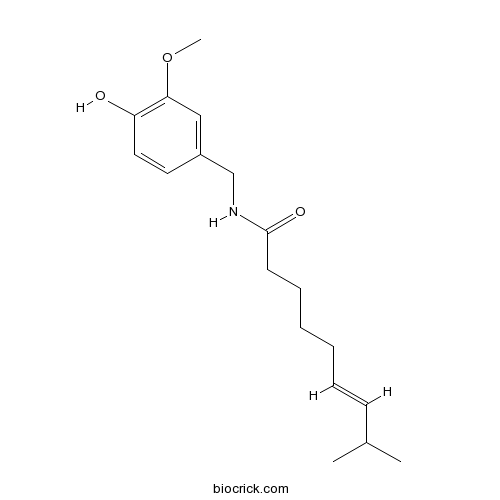
| SMILES | CC(C)C=CCCCCC(=O)NCC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | YKPUWZUDDOIDPM-SOFGYWHQSA-N | ||
| Standard InChI | InChI=1S/C18H27NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h6,8,10-12,14,20H,4-5,7,9,13H2,1-3H3,(H,19,21)/b8-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Capsaicin, the main pungent ingredient in 'hot' chilli peppers, is a TRPV1 agonist with EC50 of 0.29±0.05 μM in HEK293 cells, which elicits a sensation of burning pain by selectively activating sensory neurons that convey information about noxious stimuli to the central nervous system, it may used as a pain therapy by the long-lasting and inhibitory effects on persistent pain. Capsaicin has antioxidant activity , it is more effective than melatonin in suppressing the formation of lipid hydroperoxides, it also reduces anxiety-like behaviours in rats and may be an admissible drug candidate for treating endometriosis. |
| Targets | Akt | PI3K | mTOR | Autophagy | TRPV1 |
| In vitro | Capsaicin Enhances the Drug Sensitivity of Cholangiocarcinoma through the Inhibition of Chemotherapeutic-Induced Autophagy.[Pubmed: 25933112]PLoS One. 2015 May 1;10(5):e0121538.Cholangiocarcinoma (CCA), a devastating cancer with a poor prognosis, is resistant to the currently available chemotherapeutic agents. Capsaicin, the major pungent ingredient found in hot red chili peppers of the genus Capsicum, suppresses the growth of several malignant cell lines. Our aims were to investigate the role and mechanism of Capsaicin with respect to the sensitivity of CCA cells to chemotherapeutic agents.
Capsaicin inhibits proliferation of endometriotic cells in vitro.[Pubmed: 18391504 ]Gynecol Obstet Invest. 2008;66(1):59-62.Treatment of immortalized stromal-like and epithelial-like endometriotic cells with Capsaicin resulted in inhibition of proliferation in a concentration-dependent manner. In addition, endometriotic cells are more sensitive to Capsaicin treatment than immortalized endometrial cells, suggesting that Capsaicin may be an admissible drug candidate for treating endometriosis. |
| In vivo | Experimental evidence for alleviating nociceptive hypersensitivity by single application of capsaicin.[Pubmed: 25896608]Mol Pain. 2015 Apr 22;11(1):22.The single application of high-concentration of Capsaicin has been used as an analgesic therapy of persistent pain. However, its effectiveness and underlying mechanisms remain to be further evaluated with experimental approaches. |
| Kinase Assay | The capsaicin receptor.[Reference: WebLink]Nature, 1997, 389(6653):816-24.Capsaicin, the main pungent ingredient in 'hot' chilli peppers, elicits a s ensation of burning pain by selectively activating sensory neurons that con vey information about noxious stimuli to the central nervous system.
|
| Animal Research | Anxiolytic efficacy of repeated oral capsaicin in rats with partial aberration of oral sensory relay to brain.[Pubmed: 25874812]Arch Oral Biol. 2015 Mar 28;60(7):989-997.This study was conducted to examine if taste over load with oral Capsaicin improves the adverse behavioural effects induced by partial aberration of oral sensory relays to brain with bilateral transections of the lingual and chorda tympani nerves.
|
| Structure Identification | J Agric Food Chem. 1999 Jul;47(7):2563-70.Quantitative HPLC determination of the antioxidant activity of capsaicin on the formation of lipid hydroperoxides of linoleic acid: a comparative study against BHT and melatonin.[Pubmed: 10552527]The antioxidant activity of Capsaicin, as compared to BHT and melatonin, was determined by the direct measurement of lipid hydroperoxides formed upon linoleic acid autoxidation initiated by AIBN.
|

Capsaicin Dilution Calculator

Capsaicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2742 mL | 16.3709 mL | 32.7418 mL | 65.4836 mL | 81.8545 mL |
| 5 mM | 0.6548 mL | 3.2742 mL | 6.5484 mL | 13.0967 mL | 16.3709 mL |
| 10 mM | 0.3274 mL | 1.6371 mL | 3.2742 mL | 6.5484 mL | 8.1854 mL |
| 50 mM | 0.0655 mL | 0.3274 mL | 0.6548 mL | 1.3097 mL | 1.6371 mL |
| 100 mM | 0.0327 mL | 0.1637 mL | 0.3274 mL | 0.6548 mL | 0.8185 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Capsaicin is an anti-proliferation agent with IC50 value of 100 μM in A172 cells.
Capsaicin is an active component of chili peppers. It selectively binds to TRPV1 which is a heat-activated calcium channel. Capsaicin causes the channel to open below 37 °C. This is why capsaicin is linked to the sensation of heat. [1] Capsaicin has been reported to possess anti-carcinogenic and anti-mutagenic activities. In human glioma A172 cells, capsaicin reduced cell viability with IC50 of ~100 μM treated for 1 day. Capsaicin inhibited cell growth and induced apoptosis through down-regulation of Bcl-2 and activation of caspase-3. Capsaicin also induced terminal differentiation, which contribute to A172 cell growth inhibition.[2] On the other hand, capsaicin reduced the basal generation of ROS, which may played a role in the induction of apoptosis by capsaicin.[3] In MCF-7 cells, Capsaicin induced cell apoptosis through the mitochondrial pathway, and subsequently caused PARP-1 cleaved by activation of caspase-7.[4] In human SCLC cell line, capsaicin displayed robust anti-proliferative activity with MTT assay. Furthermore, capsaicin (10 mg/kg body weight) significantly reduced the growth rate of established (800 mm3) H69 tumors xenotransplanted in nude mice. [5]
References:
1. M. J. Caterina, M. A. Schumacher, M. Tominaga, T. A. Rosen, J. D. Levine and D. Julius, Nature 1997, 389, 816-824.
2. Y. G. Gil and M. K. Kang, Life Sci 2008, 82, 997-1003.
3. Y. S. Lee, D. H. Nam and J. A. Kim, Cancer Lett 2000, 161, 121-130.
4. H. C. Chang, S. T. Chen, S. Y. Chien, S. J. Kuo, H. T. Tsai and D. R. Chen, Hum Exp Toxicol 2011, 30, 1657-1665.
5. K. C. Brown, T. R. Witte, W. E. Hardman, H. Luo, Y. C. Chen, A. B. Carpenter, J. K. Lau and P. Dasgupta, PLoS One 2010, 5, e10243. >
- Pamidronate
Catalog No.:BCC4693
CAS No.:40391-99-9
- 6-Methoxykaempferol 3-O-rutinoside
Catalog No.:BCN2379
CAS No.:403861-33-6
- Benzyl 2-hydroxy-6-(beta-glucosyloxy)benzoate
Catalog No.:BCN7434
CAS No.:403857-21-6
- HEMADO
Catalog No.:BCC7118
CAS No.:403842-38-6
- 10058-F4
Catalog No.:BCC1050
CAS No.:403811-55-2
- Qc 1
Catalog No.:BCC6356
CAS No.:403718-45-6
- Cannabisin H
Catalog No.:BCN3896
CAS No.:403647-08-5
- Obtusafuran methyl ether
Catalog No.:BCN8103
CAS No.:40357-59-3
- Isoneobavaisoflavone
Catalog No.:BCN3195
CAS No.:40357-43-5
- p-Menth-1-ene-3,6-diol
Catalog No.:BCN5454
CAS No.:4031-55-4
- Cassyfiline
Catalog No.:BCN4763
CAS No.:4030-51-7
- MDL 12330A hydrochloride
Catalog No.:BCC7066
CAS No.:40297-09-4
- erythro-1-Phenylpropane-1,2-diol
Catalog No.:BCN6596
CAS No.:40421-52-1
- Pinusolidic acid
Catalog No.:BCN5455
CAS No.:40433-82-7
- Kaempferol-3-O-glucorhamnoside
Catalog No.:BCN2830
CAS No.:40437-72-7
- Yatein
Catalog No.:BCN5456
CAS No.:40456-50-6
- Bursehernin
Catalog No.:BCN3040
CAS No.:40456-51-7
- Ethyl ferulate
Catalog No.:BCN1257
CAS No.:4046-02-0
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
- Z-Lys(Z)-OH
Catalog No.:BCC2762
CAS No.:405-39-0
- 4'-Demethylpodophyllotoxin
Catalog No.:BCN2625
CAS No.:40505-27-9
- Salubrinal
Catalog No.:BCC4843
CAS No.:405060-95-9
- Drechslerine A
Catalog No.:BCN7561
CAS No.:405157-84-8
Experimental evidence for alleviating nociceptive hypersensitivity by single application of capsaicin.[Pubmed:25896608]
Mol Pain. 2015 Apr 22;11:22.
The single application of high-concentration of Capsaicin has been used as an analgesic therapy of persistent pain. However, its effectiveness and underlying mechanisms remain to be further evaluated with experimental approaches. The present study provided evidence showing that the single application of Capsaicin dose-dependently alleviated nociceptive hypersensitivity, and reduced the action potential firing in small-diameter neurons of the dorsal root ganglia (DRG) in rats and mice. Pre-treatment with Capsaicin reduced formalin-induced acute nocifensive behavior after a brief hyperalgesia in rats and mice. The inhibitory effects of Capsaicin were calcium-dependent, and mediated by the Capsaicin receptor (transient receptor potential vanilloid type-1). We further found that Capsaicin exerted inhibitory effects on the persistent nociceptive hypersensitivity induced by peripheral inflammation and nerve injury. Thus, these results support the long-lasting and inhibitory effects of topical Capsaicin on persistent pain, and the clinic use of Capsaicin as a pain therapy.
Quantitative HPLC determination of the antioxidant activity of capsaicin on the formation of lipid hydroperoxides of linoleic acid: a comparative study against BHT and melatonin.[Pubmed:10552527]
J Agric Food Chem. 1999 Jul;47(7):2563-70.
The antioxidant activity of Capsaicin, as compared to BHT and melatonin, was determined by the direct measurement of lipid hydroperoxides formed upon linoleic acid autoxidation initiated by AIBN. The formation of four isomeric lipid hydroperoxides was detected after reverse-phase HPLC separation. Data from three detectors, UV absorption, glassy carbon electrode electrochemical detection, and postcolumn chemiluminescence using luminol, were compared. Capsaicin was more effective than melatonin in suppressing the formation of lipid hydroperoxides but not as effective as BHT. The formation of Capsaicin and BHT dimers was observed during oxidation, and the dimers were characterized using APCI MS(n).
Anxiolytic efficacy of repeated oral capsaicin in rats with partial aberration of oral sensory relay to brain.[Pubmed:25874812]
Arch Oral Biol. 2015 Jul;60(7):989-97.
OBJECTIVE: This study was conducted to examine if taste over load with oral Capsaicin improves the adverse behavioural effects induced by partial aberration of oral sensory relays to brain with bilateral transections of the lingual and chorda tympani nerves. DESIGN: Male Sprague-Dawley rats received daily 1 ml of 0.02% Capsaicin or water drop by drop into the oral cavity following the bilateral transections of the lingual and chorda tympani nerves. Rats were subjected to ambulatory activity, elevated plus maze and forced swim tests after 11th, 14th and 17th daily administration of Capsaicin or water, respectively. The basal and stress-induced plasma corticosterone levels were examined after the end of behavioural tests. RESULTS: Ambulatory counts, distance travelled, centre zone activities and rearing were increased, and rostral grooming decreased, during the activity test in Capsaicin treated rats. Behavioural scores of Capsaicin rats during elevated plus maze test did not differ from control rats. Immobility during the swim test was decreased in Capsaicin rats with near significance (P = 0.0547). Repeated oral Capsaicin increased both the basal level and stress-induced elevation of plasma corticosterone in rats with bilateral transections of the lingual and chorda tympani nerves. DISCUSSION: It is concluded that repeated oral administration of Capsaicin reduces anxiety-like behaviours in rats that received bilateral transections of the lingual and chorda tympani nerves, and that the increased corticosterone response, possibly modulating the hippocampal neural plasticity, may be implicated in the anxiolytic efficacy of oral Capsaicin.
Capsaicin Enhances the Drug Sensitivity of Cholangiocarcinoma through the Inhibition of Chemotherapeutic-Induced Autophagy.[Pubmed:25933112]
PLoS One. 2015 May 1;10(5):e0121538.
Cholangiocarcinoma (CCA), a devastating cancer with a poor prognosis, is resistant to the currently available chemotherapeutic agents. Capsaicin, the major pungent ingredient found in hot red chili peppers of the genus Capsicum, suppresses the growth of several malignant cell lines. Our aims were to investigate the role and mechanism of Capsaicin with respect to the sensitivity of CCA cells to chemotherapeutic agents. The effect of Capsaicin on CCA tumor sensitivity to 5-fluorouracil (5-FU) was assessed in vitro in CCA cells and in vivo in a xenograft model. The drug sensitivity of QBC939 to 5-FU was significantly enhanced by Capsaicin compared with either agent alone. In addition, the combination of Capsaicin with 5-FU was synergistic, with a combination index (CI) < 1, and the combined treatment also suppressed tumor growth in the CCA xenograft to a greater extent than 5-FU alone. Further investigation revealed that the autophagy induced by 5-FU was inhibited by Capsaicin. Moreover, the decrease in AKT and S6 phosphorylation induced by 5-FU was effectively reversed by Capsaicin, indicating that Capsaicin inhibits 5-FU-induced autophagy by activating the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway in CCA cells. Taken together, these results demonstrate that Capsaicin may be a useful adjunct therapy to improve chemosensitivity in CCA. This effect likely occurs via PI3K/AKT/mTOR pathway activation, suggesting a promising strategy for the development of combination drugs for CCA.
Capsaicin inhibits proliferation of endometriotic cells in vitro.[Pubmed:18391504]
Gynecol Obstet Invest. 2008;66(1):59-62.
Treatment of immortalized stromal-like and epithelial-like endometriotic cells with Capsaicin resulted in inhibition of proliferation in a concentration-dependent manner. In addition, endometriotic cells are more sensitive to Capsaicin treatment than immortalized endometrial cells, suggesting that Capsaicin may be an admissible drug candidate for treating endometriosis.
Cannabinoid activation of recombinant and endogenous vanilloid receptors.[Pubmed:11672565]
Eur J Pharmacol. 2001 Jul 27;424(3):211-9.
The effects of three structurally related cannabinoids on human and rat recombinant vanilloid VR1 receptors expressed in human embryonic kidney (HEK293) cells and at endogenous vanilloid receptors in the rat isolated mesenteric arterial bed were studied. In the recombinant cells, all three were full agonists, causing concentration-dependent increases in [Ca(2+)](i) (FLIPR), with a rank order of potency relative to the vanilloids Capsaicin and olvanil, of olvanil> or =Capsaicin>AM404 ((allZ)-N-(4-hydroxyphenyl)-5,8,11,14-eicosatetraenamide)>anandamide>methanandami de. These responses were inhibited by the vanilloid VR1 receptor antagonist, capsazepine. In the mesenteric arterial bed, vasorelaxation was evoked by these ligands with a similar order of potency. The AM404-induced vasorelaxation was virtually abolished by Capsaicin pretreatment. AM404 inhibition of Capsaicin-sensitive sensory neurotransmission was blocked by ruthenium red, but not by cannabinoid CB(1) and CB(2) receptor antagonists. AM404 had no effect on relaxations to calcitonin gene-related peptide. These data demonstrate that the vasorelaxant and sensory neuromodulator properties of AM404 in the rat isolated mesenteric arterial bed are mediated by vanilloid VR1 receptors.
Inhibition of platelet aggregation by capsaicin. An effect unrelated to actions on sensory afferent neurons.[Pubmed:1786800]
Eur J Pharmacol. 1991 Sep 4;202(1):129-31.
The effects of Capsaicin on the ability of platelets to aggregate in response to thrombin, platelet-activating factor or calcium ionophore (A23187) were examined. At concentrations previously shown to activate sensory afferent neurons, Capsaicin markedly inhibited the responsiveness of platelets to the three agonists. The effects of Capsaicin on platelet aggregation were reversible, and could be observed if Capsaicin was added after platelets had begun to aggregate in response to the agonist. Capsaicin did not affect the shape change which occurs in response to the agonists, a process which is calcium-independent. These results demonstrate that Capsaicin, at concentrations which are frequently used to 'selectively' activate sensory afferent neurons, is also capable of affecting the function of the platelet. Such non-specific effects of Capsaicin must be considered when this substance is used as a pharmacological probe of sensory afferent nerve function.
Sensory neuron-specific actions of capsaicin: mechanisms and applications.[Pubmed:2203194]
Trends Pharmacol Sci. 1990 Aug;11(8):330-3.
Capsaicin acts specifically on a subset of primary afferent sensory neurons to open cation-selective ion channels, probably by interacting directly with a membrane receptor-ion channel complex. Another plant product--resiniferatoxin--has structural similarities to Capsaicin and opens the same channels, but is up to 10,000 times as potent. Capsaicin-sensitive neurons are involved in nociception, are responsible for the neurogenic component of the inflammatory response and may also have efferent actions in the peripheral target tissues. In addition to its excitatory actions, Capsaicin can have subsequent antinociceptive and anti-inflammatory effects. For these reasons Stuart Bevan and Janos Szolcsanyi argue that drugs based on Capsaicin and resiniferatoxin may have important clinical uses.


