HEMADOHigh affinity selective A3 agonist CAS# 403842-38-6 |
- Romidepsin (FK228, depsipeptide)
Catalog No.:BCC3597
CAS No.:128517-07-7
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 403842-38-6 | SDF | Download SDF |
| PubChem ID | 10981286 | Appearance | Powder |
| Formula | C17H23N5O4 | M.Wt | 361.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
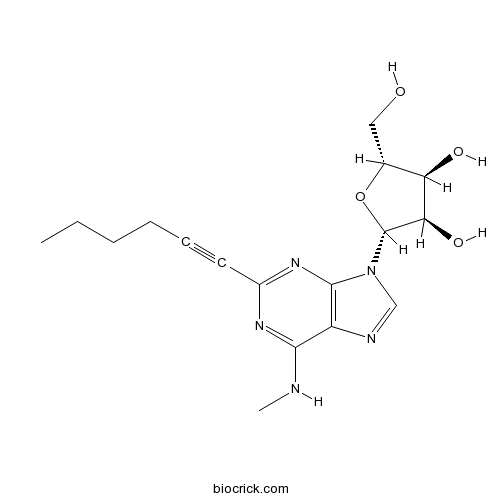
| Chemical Name | (2R,3R,4S,5R)-2-[2-hex-1-ynyl-6-(methylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol | ||
| SMILES | CCCCC#CC1=NC2=C(C(=N1)NC)N=CN2C3C(C(C(O3)CO)O)O | ||
| Standard InChIKey | KOCIMZNSNPOGOP-IWCJZZDYSA-N | ||
| Standard InChI | InChI=1S/C17H23N5O4/c1-3-4-5-6-7-11-20-15(18-2)12-16(21-11)22(9-19-12)17-14(25)13(24)10(8-23)26-17/h9-10,13-14,17,23-25H,3-5,8H2,1-2H3,(H,18,20,21)/t10-,13-,14-,17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity and selective adenosine A3 receptor agonist (Ki values are 1.1, 327, 1230 and > 30,000 nM for human A3, A1, A2A and A2B receptors respectively). |

HEMADO Dilution Calculator

HEMADO Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.767 mL | 13.8351 mL | 27.6702 mL | 55.3403 mL | 69.1754 mL |
| 5 mM | 0.5534 mL | 2.767 mL | 5.534 mL | 11.0681 mL | 13.8351 mL |
| 10 mM | 0.2767 mL | 1.3835 mL | 2.767 mL | 5.534 mL | 6.9175 mL |
| 50 mM | 0.0553 mL | 0.2767 mL | 0.5534 mL | 1.1068 mL | 1.3835 mL |
| 100 mM | 0.0277 mL | 0.1384 mL | 0.2767 mL | 0.5534 mL | 0.6918 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 10058-F4
Catalog No.:BCC1050
CAS No.:403811-55-2
- Qc 1
Catalog No.:BCC6356
CAS No.:403718-45-6
- Cannabisin H
Catalog No.:BCN3896
CAS No.:403647-08-5
- Obtusafuran methyl ether
Catalog No.:BCN8103
CAS No.:40357-59-3
- Isoneobavaisoflavone
Catalog No.:BCN3195
CAS No.:40357-43-5
- p-Menth-1-ene-3,6-diol
Catalog No.:BCN5454
CAS No.:4031-55-4
- Cassyfiline
Catalog No.:BCN4763
CAS No.:4030-51-7
- MDL 12330A hydrochloride
Catalog No.:BCC7066
CAS No.:40297-09-4
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- DR 4485 hydrochloride
Catalog No.:BCC7990
CAS No.:402942-53-4
- 7-Methoxyneochamaejasmine A
Catalog No.:BCN3134
CAS No.:402828-38-0
- SB 399885 hydrochloride
Catalog No.:BCC7595
CAS No.:402713-81-9
- Benzyl 2-hydroxy-6-(beta-glucosyloxy)benzoate
Catalog No.:BCN7434
CAS No.:403857-21-6
- 6-Methoxykaempferol 3-O-rutinoside
Catalog No.:BCN2379
CAS No.:403861-33-6
- Pamidronate
Catalog No.:BCC4693
CAS No.:40391-99-9
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- erythro-1-Phenylpropane-1,2-diol
Catalog No.:BCN6596
CAS No.:40421-52-1
- Pinusolidic acid
Catalog No.:BCN5455
CAS No.:40433-82-7
- Kaempferol-3-O-glucorhamnoside
Catalog No.:BCN2830
CAS No.:40437-72-7
- Yatein
Catalog No.:BCN5456
CAS No.:40456-50-6
- Bursehernin
Catalog No.:BCN3040
CAS No.:40456-51-7
- Ethyl ferulate
Catalog No.:BCN1257
CAS No.:4046-02-0
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
Effect of HEMADO on Level of CK-MB and LDH Enzymes after Ischemia/Reperfusion Injury in Isolated Rat Heart.[Pubmed:23878794]
Bioimpacts. 2013;3(2):101-4.
INTRODUCTION: Ischemia/Reperfusion (IR) injury mainly causes the increase of enzymes involved in myocytes injury including CK-MB (creatine kinase-MB) isoenzyme and LDH (lactate dehydrogenase). Leakage of CK-MB isoenzyme and LDH from myocardial tissues to blood is indicator of acute myocardial infarction. The aim of this study was to assess the effect of HEMADO on IR injury and its relationship with mitochondrial ATP-sensitive K+ channels (mitoKATP) in rat heart. METHODS: Twenty eight male Wistar rats (250-300g) were divided into four groups (seven members in each group): control (without ischemia), I/R (with ischemia+without HEMADO), ischemia received HEMADO (HEMADO), ischemia received HEMADO and 5-HD (5-hydroxydecanoate, specific mitoKATP channel blocker) (HEMADO+5-HD). The animals were anesthetized and the hearts were quickly removed and mounted on Langendorff apparatus and perfused by Krebs-Henseleit solution under constant pressure and temperature of 37 masculineC. After 20 minutes of stabilization, ischemic groups were exposed to 40 minutes of global ischemia and consecutive 90 minutes of reperfusion. RESULTS: IR injury increased the level of LDH and CK-MB in the collected coronary flow during 5 minutes since start of reperfusion. HEMADO reduced the enzymes' levels and using 5-HD abolished the effect of HEMADO. CONCLUSION: Our findings indicated that HEMADO could protect the heart against ischemia-reperfusion injury by decreasing the CK-MB and LDH levels. The cardioprotective effect of HEMADO may be mediated in part by mitoKATP.
[3H]HEMADO--a novel tritiated agonist selective for the human adenosine A3 receptor.[Pubmed:17126322]
Eur J Pharmacol. 2007 Feb 5;556(1-3):14-8.
Adenosine A(3) receptors are promising drug targets for a number of conditions like inflammatory diseases including asthma, ischemic injury or certain types of cancer. Consequently, intense efforts are dedicated to the development of selective A(3) agonists and antagonists. The only tritiated agonist that is available for radioligand binding is the nonselective [(3)H]5'-N-ethylcarboxamidoadenosine ([(3)H]NECA). Based on a recently characterized series of 2-substituted adenosine receptor agonists we developed a tritiated selective A(3) radioligand with high affinity. From this series 2-hexyn-1-yl-N(6)-methyladenosine (HEMADO) with a K(i)-value of 1.1 nM at the human A(3) subtype was chosen. HEMADO is 300-fold selective versus the A(1) subtype, and 1100-fold and more than 25,000-fold selective compared to the adenosine A(2A) and A(2B) receptors, respectively. The tritiated derivative [(3)H]HEMADO exhibited the same affinity as the unlabeled precursor. In concentrations up to 10 nM no specific binding to adenosine A(1), A(2A) or A(2B) receptors was observed confirming the high selectivity for adenosine A(3) receptors. Characterization of [(3)H]HEMADO in radioligand binding studies revealed reversible binding to the human adenosine A(3) subtype. In saturation binding studies for the A(3) subtype a K(D)-value of 1.1 nM was determined. Nonspecific binding at a radioligand concentration of 1 nM amounted to 1-2% of total binding. Competition binding with a panel of adenosine receptor ligands clearly confirmed the correct A(3) pharmacology of the binding site labeled by [(3)H]HEMADO. With [(3)H]HEMADO we present a tritiated agonist with high affinity and A(3)-selectivity and very low nonspecific binding. [(3)H]HEMADO is a useful tool for specific screening for A(3) receptor agonists and antagonists in improved radioligand binding assays with the human subtype.
N(6)-alkyl-2-alkynyl derivatives of adenosine as potent and selective agonists at the human adenosine A(3) receptor and a starting point for searching A(2B) ligands.[Pubmed:12109910]
J Med Chem. 2002 Jul 18;45(15):3271-9.
A series of N(6)-alkyl-2-alkynyl derivatives of adenosine (Ado) have been synthesized and evaluated for their affinity at human A(1), A(2A), and A(3) receptors and for their potency at A(2B) adenosine receptor subtypes. The corresponding 2-(1-alkynyl) derivatives of 5'-N-ethylcarboxamidoadenosine (NECA) and Ado are used as reference compounds. Binding studies demonstrated that the activities of 2-alkynylAdos were slightly increased for the adenosine A(1) receptor and slightly decreased for both A(3) and A(2B) subtypes compared to those of their corresponding NECA derivatives, whereas the A(2A) receptor affinities of the two series of nucleosides were similar. The presence of a methyl group on N(6) of the 2-alkynyladenosines, inducing an increase in affinity at the human A(3) receptor and a decrease at the other subtypes, resulted in an increase in A(3) selectivity. In particular, 2-phenylethynyl-N(6)-methylAdo (8b) showed an A(3) affinity in the low nanomolar range (K(i)(A(3)) = 3.4 nM), with a A(1)/A(3) and A(2A)/A(3) selectivity of about 500 and 2500, respectively. These findings motivated us to search for the preparation of new selective radioligands for the A(3) subtype; hence, a procedure to introduce a tritiated alkylamino group in these molecules was carried out. As far as the potency at the A(2B) receptor, the type of 2-alkynyl chain and the presence of the ethylcarboxamido group on the sugar seem to be very important; in fact, the (S)-2-phenylhydroxypropynylNECA [(S)-PHPNECA, 1e, EC(50)(A(2B)) = 0.22 microM] proved to be one of the most potent A(2B) agonist reported so far. On the other hand, the (S)-2-phenylhydroxypropynyl-N(6)-ethylAdo (9e, EC(50)(A(2B)) = 0.73 microM) showed a significantly increase of potency at the A(2B) subtype in comparison with the N(6)-methyl, N(6)-isopropyl, and the unsubstituted adenosine derivatives, although it resulted in being less potent than (S)-PHPNECA (1e, EC(50)(A(2B)) = 0.22 microM). These observations suggest that the introduction of an ethyl group in the N(6)-position and an ethylcarboxamido substituent in the 4'-position of (S)-2-phenylhydroxypropynyladenosine could lead to a compound endowed with high potency at the A(2B) receptor.


