LY 294002Potent PI3K inhibitor CAS# 154447-36-6 |
- WYE-125132 (WYE-132)
Catalog No.:BCC4608
CAS No.:1144068-46-1
- A66
Catalog No.:BCC3715
CAS No.:1166227-08-2
- PIK-75
Catalog No.:BCC1163
CAS No.:372196-77-5
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- PIK-294
Catalog No.:BCC4995
CAS No.:900185-02-6
- PI-3065
Catalog No.:BCC5379
CAS No.:955977-50-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154447-36-6 | SDF | Download SDF |
| PubChem ID | 3973 | Appearance | Powder |
| Formula | C19H17NO3 | M.Wt | 307.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 697286; SF 1101 | ||
| Solubility | DMSO : 41.67 mg/mL (135.58 mM; Need ultrasonic) | ||
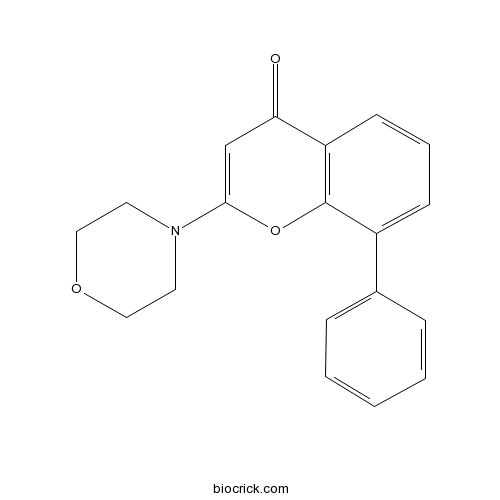
| Chemical Name | 2-morpholin-4-yl-8-phenylchromen-4-one | ||
| SMILES | C1COCCN1C2=CC(=O)C3=C(O2)C(=CC=C3)C4=CC=CC=C4 | ||
| Standard InChIKey | CZQHHVNHHHRRDU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LY294002 is an inhibitor of PI3Kα/δ/β with IC50 of 0.5 μM/0.57 μM/0.97 μM, respectively. | |||||
| Targets | p110α | p110δ | p110β | |||
| IC50 | 0.5 μM | 0.57 μM | 0.97 μM | |||
| Cell experiment: [1] | |
| Cell lines | OVCAR-3 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 10 μM, 24 hours |
| Applications | After 24 h of treatment, the number of cells in 1, 5, and 10 μM LY294002-treated wells was reduced by 27, 56, and 75%, respectively, compared to the control group. LY294002 (1–10 μM) markedly inhibited cell proliferation. When the cells were treated with 10 μM LY294002 for 24 h, the effects appeared toxic. Cellularity was decreased, and the cell clusters appeared shrunken with poor cellular cohesion. Cells had hyperchromatic, pyknotic nuclei, and the amount of cytoplasm was decreased. LY294002 induced nuclear pyknosis and diminished cytoplasmic volume, which was clearly demonstrated in the 5 μM-treated wells. |
| Animal experiment: [1] | |
| Animal models | Athymic immunodeficient mice injected with OVCAR-3 cells |
| Dosage form | Intraperitoneal injection, 100 mg/kg body weight, daily for 3 weeks |
| Application | At postmortem examination, tumors were found on the surface of the peritoneum, intestines, and uterus in both treatment and control groups. However, in the control group, tumors were also found on the diaphragm and in the hilus of the liver. LY294002 induced pyknosis and nuclear condensation, as well as reduced cytoplasmic volume in the tumor cells. Some nuclei separated from the cytoplasm. Abdominal circumference (7.2 ± 2 cm) significantly increased in the control group compared to the LY294002-treated group (6.35 ± 0.42 cm). Body weight increased in both groups for the first week after inoculation. In the control group, body weight continued to increase, whereas there was no significant change in body weight after treatment with LY294002. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Hu L, Zaloudek C, Mills G B, et al. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clinical Cancer Research, 2000, 6(3): 880-886. | |

LY 294002 Dilution Calculator

LY 294002 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2537 mL | 16.2686 mL | 32.5373 mL | 65.0745 mL | 81.3431 mL |
| 5 mM | 0.6507 mL | 3.2537 mL | 6.5075 mL | 13.0149 mL | 16.2686 mL |
| 10 mM | 0.3254 mL | 1.6269 mL | 3.2537 mL | 6.5075 mL | 8.1343 mL |
| 50 mM | 0.0651 mL | 0.3254 mL | 0.6507 mL | 1.3015 mL | 1.6269 mL |
| 100 mM | 0.0325 mL | 0.1627 mL | 0.3254 mL | 0.6507 mL | 0.8134 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY294002 is a potent inhibitor of class I phosphoinositide 3-kinases (PI3Ks) [1, 2]. It is cell permeable and reversible, and selective against p110α, p110β, p110γ and p110δ, which acts on ATP binding site of the catalytic subunit.
PI3K family is divided into 3 classes: class I, II and III. LY294002 can inhibit 3 out of 4 isoforms of catalytic subunit of class I group. The IC50s for p110α, β andδ are 500 nM, 973 nM and 570 nM [3]. Even though it is less potent than wortmannin, other PI3K inhibitor, but it is more stable in solution. In addition, LY294002 is a reversible inhibitor whereas wortmannin acts irreversibly. [2]
PI3Ks signal through Akt, activates mTOR and inhibits Bad, leading to cell growth and proliferation. LY294002 potently inactivates PI3K class I catalytic subunit, resulting in induction of apoptosis and suppression of tumor cell growth in vitro and in vivo. [2, 4]
LY294002 is also a potent autophagy inhibitor by blocking autophagosome formation. [5]
Quantitative chemoproteomic profiling shows that LY294002 inhibits the BET bromodomain proteins BRD2, BRD3, and BRD4 with IC50 value of 1-2 µM. [6]
References:
[1]Maira et al. (2009). PI3K inhibitors for cancer treatment: where do we stand?. doi:10.1042/BST0370265.
[2]Vlahos CJ., et al. (1994). A Specific Inhibitor of Phosphatidylinositol 3-Kinase, 2-(4-Morpholinyl)-8-phenyl-4H-l-benzopyran-4-one (LY294002). Journal of Biological Chemistry 269 (7): 5241–5248.
[3]Chaussade C, et al. Evidence for functional redundancy of class IA PI3K isoforms in insulin signalling. Biochem J, 2007, 404(3), 449-58.
[4]Hu L, et al. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin Cancer Res, 2000, 6(3), 880-6.
[5]Blommaart EF., et al., (1997). The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 243: 240–246.
[6]Dittmann, A., et al. (2013). The Commonly Used PI3-Kinase Probe LY294002 is an Inhibitor of BET Bromodomains. ACS Chemical Biology: 131210150813004.
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- Stauprimide
Catalog No.:BCC7768
CAS No.:154589-96-5
- Efavirenz
Catalog No.:BCC4135
CAS No.:154598-52-4
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- 3-Methoxymollugin
Catalog No.:BCN7164
CAS No.:154706-44-2
- 2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol
Catalog No.:BCN1555
CAS No.:154801-30-6
- Cimiside E
Catalog No.:BCN7951
CAS No.:154822-57-8
- 4-Acetoxycinnamic acid
Catalog No.:BCN5026
CAS No.:15486-19-8
Dissimilar effects of LY 294002 and PD 098059 in IGF-I-mediated inhibition of TGF-beta1 expression and apoptosis in bovine mammary epithelial cells.[Pubmed:16077202]
J Physiol Pharmacol. 2005 Jun;56 Suppl 3:181-93.
In mammary epithelial cells (MEC) TGF-beta(1) is the auto-/paracrine growth inhibitor and inducer of apoptosis and therefore is considered as an important local regulator of mammary tissue involution. However, the mechanisms of controlled TGF-beta(1) expression in the course of bovine mammary gland remodelling are still unclear. Recent study performed in this laboratory support the evidence that TGF-beta(1) expression in bovine MEC is regulated by hormones of somatotropic axis (GH, IGF-I and somatostatin). Present study was focused on the contribution of IGF-I-induced signaling pathways in anti-TGF-beta(1) and anti-apoptotic effects of IGF-I. Laser scanning cytometry was applied for the measurement of TGF-beta(1) content and apoptotic cell number in bovine BME-UV1 MEC. Involution of the bovine mammary gland in vitro was modeled by decreasing the availability of FBS for bovine MEC. Reducing FBS content in the medium from 10% to 0.5% evoked highly significant increase of TGF-beta(1) expression and increase of apoptotic cell number. IGF-I (50 ng/ml) completely abrogated FBS deficiency-induced TGF-beta(1) expression and apoptosis in bovine MEC. In order to establish which of the IGF-I signaling pathways contributed to anti-TGF-beta(1) and anti-apoptotic effects, the inhibitors of PI3-kinase - (LY 294002) and MEK- (MAPKK for ERK) (PD 098059) mediated signaling pathways were applied to our model. The results clearly showed that inhibition of PI3-K reverses the ability of IGF-I to suppress TGF-beta(1) expression and apoptosis. An inhibition of ERK1/2 pathway even potentiated inhibitory effect of IGF-I on TGF-beta(1) expression, but partially abrogated anti-apoptotic effect of IGF-I. In conclusion, the results of the study indicate that PI3-K/Akt pathway contributed significantly to the inhibition of TGF-beta(1) expression by IGF-I, whereas both PI3-K/Akt and ERK1/2 pathways are involved in the anti-apoptotic effect of IGF-I in bovine MEC.
The phosphatidylinositol 3-kinase inhibitor LY 294002 inhibits GlyT1-mediated glycine uptake.[Pubmed:18621031]
Brain Res. 2008 Aug 28;1227:42-51.
The actions of neurotransmitter glycine are regulated by the Na+/Cl(-) dependent high-affinity glycine transporters, GlyT1 and GlyT2. These two members of the SLC6 transport family have been cloned and extensively characterized, however relatively little is known regarding their modulation. In the present study, glycine uptake in primary cultures of rat embryonic cortex has been characterized and the effects of the phosphatidylinositol 3 (PI3) kinase inhibitors LY 294002 and wortmannin on GlyT1- and GlyT2-mediated glycine uptake were investigated. GlyT1 inhibitors ALX 5407 and sarcosine reduced total glycine uptake to 80% whereas the specific GlyT2 inhibitor Org 25543 had no effect. In the presence of alanine, glycine uptake was completely blocked by the GlyT1 inhibitors ALX 5407 and sarcosine, suggesting that the high-affinity glycine uptake occurs predominantly via GlyT1. Kinetic analysis of GlyT1 revealed the Km value of 27+/-1.5 microM and Vmax value of 157+/-14 pmol/mg/min. LY 294002, a PI3 kinase inhibitor, blocked the GlyT1-mediated glycine uptake with an IC50 value of 81+/-2 microM, whereas another inhibitor wortmannin did not show any effect. In human placental choriocarcinoma (JAR) cells, which have been previously shown to predominantly express GlyT1a, LY 294002 showed a similar potency with an IC50 value of 86+/-3 microM. Immunoblots demonstrated that LY 294002 and wortmannin inhibited PI3 kinase-dependent Akt phosphorylation in the primary cultures with IC50 values of 10+/-4 microM and 7+/-1 nM, respectively. These results suggest that the commonly used PI3 kinase blocker LY 294002 may modulate GlyT1 function independent of PI3 kinase inhibition. Kinetic analysis in the presence of LY 294002 demonstrated significant decreases of both Km and Vmax values, suggesting a mechanism of uncompetitive inhibition on GlyT1-mediated glycine uptake. In addition, glycine release was blocked by LY 294002. These results raised a possibility that LY 294002 might interact with GlyT1.
LY 294002 inhibits adenosine receptor activation by a mechanism independent of effects on PI-3 kinase or casein kinase II.[Pubmed:18404524]
Purinergic Signal. 2005 Dec;1(4):389-94.
Adenosine reduces both evoked and spontaneous calcium-dependent acetylcholine (ACh) release through a mechanism downstream of calcium entry at amphibian motor nerve endings (Silinsky EM. J Physiol 1984; 346: 243-56). LY 294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one), an inhibitor of both phosphoinositide-3 kinase (PI-3 kinase) and casein kinase II, has been reported to increase spontaneous ACh release reflected in miniature endplate potential (MEPP) frequencies independently of intraterminal calcium at the frog neuromuscular junction (Rizzoli SO, Betz WJ. J Neurosci 2002; 22: 10680-9). It has been suggested that the increase in MEPP frequency caused by LY 294002, is mediated through an action on synaptotagmins, vesicle associated calcium sensors believed to trigger synaptic vesicle exocytosis. We thus examined the effects of adenosine on MEPP frequencies and evoked ACh release reflected as endplate potentials (EPPs) in order to determine if the presumed calcium-independent ACh release is affected by adenosine. We also wanted to determine if PI-3 kinase or casein kinase II is involved in mediating or modulating the inhibitory effects of adenosine. To these ends, we examined the effects of adenosine in the presence of LY 294002, wortmannin (a highly selective the PI-3 kinase inhibitor), or DRB (5,6-dichlorobenzimidazole riboside, an inhibitor of casein kinase II). LY 294002 reduced the sensitivity of both MEPP frequencies and the nerve-evoked calcium dependent EPPs to adenosine. The occlusive effects of LY 294002 on the actions of adenosine on MEPPs and EPPs were overcome by increasing adenosine concentration. Neither wortmannin nor DRB had any effect on the sensitivity of the EPPs to adenosine indicating that neither PI-3 kinase nor casein kinase II inhibition mediates the reduction in motor-nerve terminal sensitivity to adenosine produced by LY 294002. The results indicate a competitive relationship between LY 294002 and adenosine at A(1) receptors at the frog neuromuscular junction. This effect is independent of the previously described effects of LY 294002 on the exocytotic process, and is also independent of PI-3 kinase or casein kinase II.
Phosphatidylinositol-3-kinase inhibitor LY 294002 blocks Streptococcus mutans-induced interleukin (IL)-6 and IL-8 gene expression in odontoblast-like cells.[Pubmed:18637849]
Int Endod J. 2008 Sep;41(9):763-71.
AIM: To investigate the involvement of the phosphatidylinositol-3-kinase (PI3K) in interleukin-6 (IL-6) and interleukin-8 (IL-8) gene expression in odontoblast-like cells when exposed to heat-killed Streptococcus mutans. METHODOLOGY: Cultured human odontoblast-like cells (Dulbecco's modified Eagle's medium) were pre-treated with a specific inhibitor for PI3K (LY 294002) and subsequently stimulated with heat-killed S. mutans for 6 and 24 h. After stimulation, RNA was extracted from the cells and cDNA synthesis was performed. Gene expression of IL-6 and IL-8 was analysed by real-time polymerase chain reaction and normalized to the gene expression of beta-actin. Cell survival was determined for stimulation experiments. RESULTS: The gene expression of IL-6 and IL-8 was significantly increased in response to heat-killed S. mutans (P = 0.002 and P < 0.001, respectively). After 6 h, the mRNA expression of IL-6 and IL-8 was significantly higher than after 24 h of stimulation (P = 0.019 and P < 0.001, respectively). Pre-treatment with the inhibitor LY 294002 blocked the induced gene expression of IL-6 and IL-8 (P = 0.002 and P < 0.001, respectively). No differences in viable cell counts were found after stimulation with heat-killed S. mutans and/or pre-treatment with LY 294002 compared with the unstimulated control. CONCLUSION: Gene expression of IL-6 and IL-8 was induced by heat-killed S. mutans via signalling pathways mediated by PI3K. These findings indicate that odontoblasts respond to cariogenic bacteria with increased gene expression of pro-inflammatory mediators and hence participate in immune processes.


