A66P110α inhibitor CAS# 1166227-08-2 |
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1166227-08-2 | SDF | Download SDF |
| PubChem ID | 42636535 | Appearance | Powder |
| Formula | C17H23N5O2S2 | M.Wt | 393.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (127.06 mM; Need ultrasonic) | ||
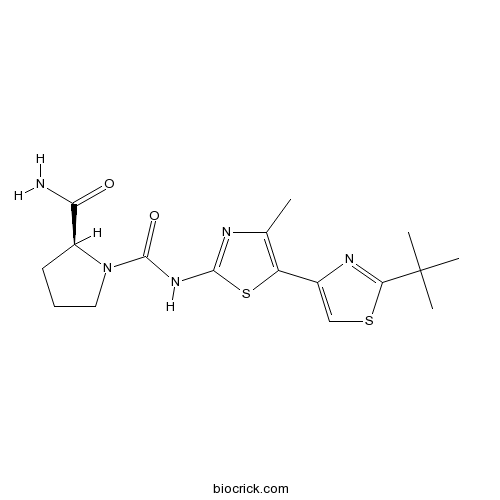
| Chemical Name | (2S)-1-N-[5-(2-tert-butyl-1,3-thiazol-4-yl)-4-methyl-1,3-thiazol-2-yl]pyrrolidine-1,2-dicarboxamide | ||
| SMILES | CC1=C(SC(=N1)NC(=O)N2CCCC2C(=O)N)C3=CSC(=N3)C(C)(C)C | ||
| Standard InChIKey | HBPXWEPKNBHKAX-NSHDSACASA-N | ||
| Standard InChI | InChI=1S/C17H23N5O2S2/c1-9-12(10-8-25-14(20-10)17(2,3)4)26-15(19-9)21-16(24)22-7-5-6-11(22)13(18)23/h8,11H,5-7H2,1-4H3,(H2,18,23)(H,19,21,24)/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective PI 3-kinase p110α inhibitor (IC50 = 32 nM). Exhibits >100-fold selectivity for p110α over other PI 3-kinase isoforms. Inhibits Akt signaling and tumor growth in SK-OV-3 xenografts in mice. |

A66 Dilution Calculator

A66 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5411 mL | 12.7055 mL | 25.411 mL | 50.822 mL | 63.5276 mL |
| 5 mM | 0.5082 mL | 2.5411 mL | 5.0822 mL | 10.1644 mL | 12.7055 mL |
| 10 mM | 0.2541 mL | 1.2706 mL | 2.5411 mL | 5.0822 mL | 6.3528 mL |
| 50 mM | 0.0508 mL | 0.2541 mL | 0.5082 mL | 1.0164 mL | 1.2706 mL |
| 100 mM | 0.0254 mL | 0.1271 mL | 0.2541 mL | 0.5082 mL | 0.6353 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A66 is a potent and selective p110α inhibitor with IC50 of 32 nM.
P110α is the catalytic subunit of a class I Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic that is composed of a 85 kDa regulatory subunit and a 110 kDa catalytic subunit.
A66 treatment inhibits phosphorylation of Akt/PKB in certain cell lines that have H1047R mutation in PIK3CA and high levels of p110a [1]. A66 prolonged treatment reduced phosphorylation of S6K on Thr389, a marker of activity of this kinase and prevent IRS downregulation induced by chronic insulin treatment in both C2C12 myoblasts and 3T3-L1 adipocytes [2].
The component has also been used extensively in various animal models to study the role of PI3K. For instance, inhibition of p110α by A66 treatment was shown to reduce tumor growth of certain cell lines in in vivo xenograft models [1]. Chronic pharmacological inhibition of p110α through A66 treatment protects from insulin resistance induced by sustained metabolic stress [2]. In the mouse model of mammary fat pad transplantation of athymic mice with primary tumors, treatment of transplanted tumor-bearing mice with A66 reduce tumor growth dramatically [3].
References:
1.Jamieson S, Flanagan JU, Kolekar S, Buchanan C, Kendall JD, Lee WJ, et al. A drug targeting only p110alpha can block phosphoinositide 3-kinase signalling and tumour growth in certain cell types. Biochem J 2011,438:53-62.
2.Foukas LC, Bilanges B, Bettedi L, Pearce W, Ali K, Sancho S, et al. Long-term p110alpha PI3K inactivation exerts a beneficial effect on metabolism. EMBO Mol Med 2013,5:563-571.
3.Utermark T, Rao T, Cheng H, Wang Q, Lee SH, Wang ZC, et al. The p110alpha and p110beta isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev 2012,26:1573-1586.
- 3-Methylamino-1-(2-thienyl)-1-propanol
Catalog No.:BCC8636
CAS No.:116539-55-0
- Dehydroalisol B 23-acetate
Catalog No.:BCC9240
CAS No.:
- Aflatoxin G1
Catalog No.:BCC9214
CAS No.:1165-39-5
- 9alpha,13alpha-Epidioxyabiet-8(14)-en-18-oic acid
Catalog No.:BCN1611
CAS No.:116499-73-1
- 5,5'-Dimethoxylariciresinol
Catalog No.:BCN6043
CAS No.:116498-58-9
- ML 145
Catalog No.:BCC7876
CAS No.:1164500-72-4
- Sal 003
Catalog No.:BCC2465
CAS No.:1164470-53-4
- Curcumadione
Catalog No.:BCN3525
CAS No.:116425-36-6
- Aerugidiol
Catalog No.:BCN3529
CAS No.:116425-35-5
- Fargesone B
Catalog No.:BCN6415
CAS No.:116424-70-5
- Fargesone A
Catalog No.:BCN6417
CAS No.:116424-69-2
- 9S-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3080
CAS No.:1164201-85-7
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Mycophenolate mofetil hydrochloride
Catalog No.:BCC4117
CAS No.:116680-01-4
- AZD7687
Catalog No.:BCC1394
CAS No.:1166827-44-6
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- H-9 dihydrochloride
Catalog No.:BCC5656
CAS No.:116700-36-8
- Glycyrrhisoflavone
Catalog No.:BCN2930
CAS No.:116709-70-7
- Novaluron
Catalog No.:BCC5466
CAS No.:116714-46-6
- 9'-Methyl lithospermate B
Catalog No.:BCN2824
CAS No.:1167424-31-8
- 9'''-Methyl salvianolate B
Catalog No.:BCN2923
CAS No.:1167424-32-9
- 2-(4-Hydroxyphenyl)-6-methyl-2,3-dihydro-4H-pyran-4-one
Catalog No.:BCN1610
CAS No.:1167483-18-2
- 4',5,6,7-Tetramethoxyflavone
Catalog No.:BCN8256
CAS No.:1168-42-9
Draft Genome Sequence of the Streptococcus pneumoniae Avery Strain A66.[Pubmed:26112793]
Genome Announc. 2015 Jun 25;3(3). pii: 3/3/e00697-15.
We have used HiSeq 2000 technology to generate a draft genome sequence of Streptococcus pneumoniae strain A66. This is a common study strain used in investigations of pneumococcal bacterium-host interactions and was used in the seminal genetic studies of Avery et al.
Variation in hormone autonomy and regenerative potential of cells transformed by strain A66 of Agrobacterium tumefaciens.[Pubmed:6297775]
Cell. 1982 Dec;31(3 Pt 2):605-12.
Mutant Agrobacterium tumefaciens strain A66 is shown to differ from its wild-type progenitor (strain A6) by a spontaneous 2.7 kb DNA insert into the T-DNA region of its Ti plasmid. Tobacco stems transformed by A66 exhibit an attenuated response characterized by slow growth and shoot proliferation. Clonal analysis demonstrates that this response is due to an alteration in the growth and regenerative potential of transformed cells, rather than to variation in the frequency of fully autonomous cells within the primary tumor. Cloned A66 transformed tobacco cells exhibit an auxin requirement for growth that can be overcome by shoot proliferation. Other host species, however, may complement the A66 mutation yielding fully auxin-independent tumors when transformed by this bacterium.
Compensation for a Mutated Auxin Biosynthesis Gene of Agrobacterium Ti Plasmid A66 in Nicotiana glutinosa Does Not Result from Increased Auxin Accumulation.[Pubmed:16666706]
Plant Physiol. 1989 Apr;89(4):1337-40.
Nicotiana glutinosa compensated for a mutated tumor-morphology-shooty (tms) (auxin biosynthesis) locus of Agrobacterlum tumefaciens strain A66 and showed the same virulent tumor response to infection by strain A66 or the wild-type strain A6. Cloned cell lines transformed by strains A6 or A66 were fully hormone independent in culture and grew rapidly as friable, unorganized tissues on hormone-free growth medium. Growth of N. glutinosa tumor cells was inhibited by addition of alpha-naphthaleneacetic acid to the growth medium, and A6- and A66-transformed cells showed similar dose responses to this auxin. On the other hand, A6-transformed cells contained much higher levels of indole-3-acetic acid (IAA) and 1-aminocyclopropane-1-carboxylic acid (ACC) than A66-transformed cells. Differences in IAA and ACC levels in N. glutinosa tumor lines were consistent with the expected activity of the tms locus and were quantitatively similar to results obtained previously with A6- and A66-transformed cells of Nicotiana tabacum, which does not compensate for mutated tms genes. Thus, compensation for mutated tms genes in N. glutinosa did not result from increased auxin accumulation and did not appear to be related to the capacity of this host for auxin biosynthesis.
Inhibition of Vibrio biofilm formation by a marine actinomycete strain A66.[Pubmed:17624525]
Appl Microbiol Biotechnol. 2007 Oct;76(5):1137-44.
China remains by far the largest aquaculture producer in the world. However, biofilms formed by pathogenic Vibrio strains pose serious problems to marine aquaculture. To provide a strategy for biofilm prevention, control, and eradication, extracts from 88 marine actinomycetes were screened. Thirty-five inhibited the biofilm formation of Vibrio harveyi, Vibrio vulnificus, and Vibrio anguillarum at a concentration of 2.5% (v/v). Thirty-three of the actinomycete extracts dispersed the mature biofilm. Six extracts inhibited the quorum-sensing system of V. harveyi by attenuating the signal molecules N-acylated homoserine lactones' activity. Strain A66, which was identified as Streptomyces albus, both attenuated the biofilms and inhibited their quorum-sensing system. It is suggested that strain A66 is a promising candidate to be used in future marine aquaculture.
A drug targeting only p110alpha can block phosphoinositide 3-kinase signalling and tumour growth in certain cell types.[Pubmed:21668414]
Biochem J. 2011 Aug 15;438(1):53-62.
Genetic alterations in PI3K (phosphoinositide 3-kinase) signalling are common in cancer and include deletions in PTEN (phosphatase and tensin homologue deleted on chromosome 10), amplifications of PIK3CA and mutations in two distinct regions of the PIK3CA gene. This suggests drugs targeting PI3K, and p110alpha in particular, might be useful in treating cancers. Broad-spectrum inhibition of PI3K is effective in preventing growth factor signalling and tumour growth, but suitable inhibitors of p110alpha have not been available to study the effects of inhibiting this isoform alone. In the present study we characterize a novel small molecule, A66, showing the S-enantiomer to be a highly specific and selective p110alpha inhibitor. Using molecular modelling and biochemical studies, we explain the basis of this selectivity. Using a panel of isoform-selective inhibitors, we show that insulin signalling to Akt/PKB (protein kinase B) is attenuated by the additive effects of inhibiting p110alpha/p110beta/p110delta in all cell lines tested. However, inhibition of p110alpha alone was sufficient to block insulin signalling to Akt/PKB in certain cell lines. The responsive cell lines all harboured H1047R mutations in PIK3CA and have high levels of p110alpha and class-Ia PI3K activity. This may explain the increased sensitivity of these cells to p110alpha inhibitors. We assessed the activation of Akt/PKB and tumour growth in xenograft models and found that tumours derived from two of the responsive cell lines were also responsive to A66 in vivo. These results show that inhibition of p110alpha alone has the potential to block growth factor signalling and reduce growth in a subset of tumours.


