GlycyrrhisoflavoneCAS# 116709-70-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 116709-70-7 | SDF | Download SDF |
| PubChem ID | 5317764 | Appearance | Powder |
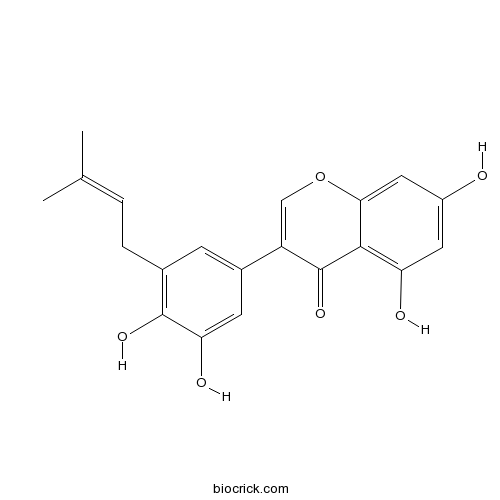
| Formula | C20H18O6 | M.Wt | 354.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[3,4-dihydroxy-5-(3-methylbut-2-enyl)phenyl]-5,7-dihydroxychromen-4-one | ||
| SMILES | CC(=CCC1=C(C(=CC(=C1)C2=COC3=CC(=CC(=C3C2=O)O)O)O)O)C | ||
| Standard InChIKey | JOQWUUJQWPZLAT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H18O6/c1-10(2)3-4-11-5-12(6-16(23)19(11)24)14-9-26-17-8-13(21)7-15(22)18(17)20(14)25/h3,5-9,21-24H,4H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glycyrrhisoflavone is a tyrosinase inhibitor, it also shows inhibitory effects on monoamine oxidase. Glycybenzofuran shows significant protein tyrosine phosphatase-1B (PTP1B) inhibitory activity in vitro with the IC50 value of 25.5 microM. Glycyrrhisoflavone has anti-inflammatory effects, it has significant inhibitory effects against NO production on LPS-induced RAW 264.7 cell model. |
| Targets | NO | MAO | Tyrosinase |
| In vitro | Rapid discovery and identification of anti-inflammatory constituents from traditional Chinese medicine formula by activity index, LC-MS, and NMR.[Pubmed: 27499135 ]Sci Rep. 2016 Aug 8;6:31000.The traditional activity-guided approach has the shortcoming of low accuracy and efficiency in discovering active compounds from TCM. |
| Kinase Assay | Phenolic constituents of licorice. III. Structures of glicoricone and licofuranone, and inhibitory effects of licorice constituents on monoamine oxidase.[Pubmed: 1913999]Identification of tyrosinase inhibitors from Glycyrrhiza uralensis.[Pubmed: 16142649]Prenylflavonoids from Glycyrrhiza uralensis and their protein tyrosine phosphatase-1B inhibitory activities.[Pubmed: 20724155 ]Bioorg Med Chem Lett. 2010 Sep 15;20(18):5398-401
Planta Med. 2005 Aug;71(8):785-7.Tyrosinase is a key enzyme in the production of melanins. Chem Pharm Bull (Tokyo). 1991 May;39(5):1238-43.Two new phenolic compounds, glicoricone (3) and licofuranone (4), were isolated from a species of licorice brought from the northwestern region of China, and their structures were assigned. |

Glycyrrhisoflavone Dilution Calculator

Glycyrrhisoflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8217 mL | 14.1084 mL | 28.2167 mL | 56.4334 mL | 70.5418 mL |
| 5 mM | 0.5643 mL | 2.8217 mL | 5.6433 mL | 11.2867 mL | 14.1084 mL |
| 10 mM | 0.2822 mL | 1.4108 mL | 2.8217 mL | 5.6433 mL | 7.0542 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5643 mL | 1.1287 mL | 1.4108 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5643 mL | 0.7054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-9 dihydrochloride
Catalog No.:BCC5656
CAS No.:116700-36-8
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- AZD7687
Catalog No.:BCC1394
CAS No.:1166827-44-6
- Mycophenolate mofetil hydrochloride
Catalog No.:BCC4117
CAS No.:116680-01-4
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- A66
Catalog No.:BCC3715
CAS No.:1166227-08-2
- 3-Methylamino-1-(2-thienyl)-1-propanol
Catalog No.:BCC8636
CAS No.:116539-55-0
- Dehydroalisol B 23-acetate
Catalog No.:BCC9240
CAS No.:
- Aflatoxin G1
Catalog No.:BCC9214
CAS No.:1165-39-5
- 9alpha,13alpha-Epidioxyabiet-8(14)-en-18-oic acid
Catalog No.:BCN1611
CAS No.:116499-73-1
- 5,5'-Dimethoxylariciresinol
Catalog No.:BCN6043
CAS No.:116498-58-9
- Novaluron
Catalog No.:BCC5466
CAS No.:116714-46-6
- 9'-Methyl lithospermate B
Catalog No.:BCN2824
CAS No.:1167424-31-8
- 9'''-Methyl salvianolate B
Catalog No.:BCN2923
CAS No.:1167424-32-9
- 2-(4-Hydroxyphenyl)-6-methyl-2,3-dihydro-4H-pyran-4-one
Catalog No.:BCN1610
CAS No.:1167483-18-2
- 4',5,6,7-Tetramethoxyflavone
Catalog No.:BCN8256
CAS No.:1168-42-9
- GDC-0623
Catalog No.:BCC4150
CAS No.:1168091-68-6
- 5-Formamide-1-(2-formyloxyethl)pyrazole
Catalog No.:BCC8747
CAS No.:116856-18-9
- Fmoc-D-Ser-OH
Catalog No.:BCC3547
CAS No.:116861-26-8
- 20-Hydroxyaflavinine
Catalog No.:BCN7283
CAS No.:116865-08-8
- Monohydroxyisoaflavinine
Catalog No.:BCN7284
CAS No.:116865-09-9
- XL413
Catalog No.:BCC4241
CAS No.:1169558-38-6
- XL413 hydrochloride
Catalog No.:BCC4039
CAS No.:1169562-71-3
Phenolic constituents of licorice. III. Structures of glicoricone and licofuranone, and inhibitory effects of licorice constituents on monoamine oxidase.[Pubmed:1913999]
Chem Pharm Bull (Tokyo). 1991 May;39(5):1238-43.
Two new phenolic compounds, glicoricone (3) and licofuranone (4), were isolated from a species of licorice brought from the northwestern region of China, and their structures were assigned. Among the twelve licorice constituents examined for the inhibition of monoamine oxidase (MAO), six compounds, 3, 4, genistein (6), licopyranocoumarin (7), licocoumarone (14) and Glycyrrhisoflavone (15), inhibited the enzyme with the IC50 (concentration required for 50% inhibition of the enzyme activity) values of 6.0 x 10(-5)-1.4 x 10(-4) M. Glycyrrhizin (1) also inhibited MAO with the IC50 value of 1.6 x 10(-4) M.
Rapid discovery and identification of anti-inflammatory constituents from traditional Chinese medicine formula by activity index, LC-MS, and NMR.[Pubmed:27499135]
Sci Rep. 2016 Aug 8;6:31000.
The traditional activity-guided approach has the shortcoming of low accuracy and efficiency in discovering active compounds from TCM. In this work, an approach was developed by integrating activity index (AI), liquid chromatography - mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR) to rapidly predict and identify the potential active constituents from TCM. This approach was used to discover and identify the anti-inflammatory constituents from a TCM formula, Gui-Zhi-Jia-Shao-Yao-Tang (GZJSYT). The AI results indicated that, among the 903 constituents detected in GZJSYT by LC-MS, 61 constituents with higher AI values were very likely to have anti-inflammatory activities. And eight potential active constituents of them were isolated and validated to have significant inhibitory effects against NO production on LPS-induced RAW 264.7 cell model. Among them, Glycyrrhisoflavone (836), glisoflavanone (893) and isoangustone A (902) were reported to have anti-inflammatory effects for the first time. The proposed approach could be generally applicable for rapid and high efficient discovery of anti-inflammatory constituents from other TCM formulae or natural products.
Identification of tyrosinase inhibitors from Glycyrrhiza uralensis.[Pubmed:16142649]
Planta Med. 2005 Aug;71(8):785-7.
Tyrosinase is a key enzyme in the production of melanins. Phytochemical studies of a Glycyrrhiza uralensis extract were performed by measuring the tyrosinase and melanin synthesis inhibitory activity. Glycyrrhisoflavone and glyasperin C were identified as tyrosinase inhibitors for the first time. Glyasperin C showed a stronger tyrosinase inhibitory activity (IC (50) = 0.13 +/- 0.01 microg/mL) than glabridin (IC (50) = 0.25 +/- 0.01 microg/mL) and a moderate inhibition of melanin production (17.65 +/- 8.8 % at 5 microg/mL). Glycyrrhisoflavone showed a strong melanin synthesis inhibitory activity (63.73 +/- 6.8 % inhibition at 5 microg/mL). These results suggest that glyasperin C and Glycyrrhisoflavone could be promising candidates in the design of skin-whitening agents.
Prenylflavonoids from Glycyrrhiza uralensis and their protein tyrosine phosphatase-1B inhibitory activities.[Pubmed:20724155]
Bioorg Med Chem Lett. 2010 Sep 15;20(18):5398-401.
Two new 2-arylbenzofurans, glycybenzofuran (1) and cyclolicocoumarone (2), together with 10 known flavonoids including licocoumarone (3), Glycyrrhisoflavone (4), glisoflavone (5), cycloGlycyrrhisoflavone (6), isoliquiritigenin (7), licoflavone A (8), apigenin (9), isokaempferide (10), glycycoumarin (11), and isoglycycoumarin (12), were isolated from the roots of Glycyrrhiza uralensis and their structures were determined by extensive spectroscopic analyses. Compounds 1 and 5 showed significant protein tyrosine phosphatase-1B (PTP1B) inhibitory activity in vitro with the IC50 values of 25.5 and 27.9 microM, respectively. The structure-activity relationship indicated that the presence of prenyl group and ortho-hydroxy group is important for exhibiting the activity. Kinetic analysis indicated that compound 1 inhibits PTP1B by a competitive mode, whereas compound 5 by a mixed mode.


