GDC-0623MEK1 inhibitor, potent and ATP-uncompetitive CAS# 1168091-68-6 |
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- Trametinib (GSK1120212)
Catalog No.:BCC1282
CAS No.:871700-17-3
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1168091-68-6 | SDF | Download SDF |
| PubChem ID | 42642654 | Appearance | Powder |
| Formula | C16H14FIN4O3 | M.Wt | 456.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 30 mg/mL (65.76 mM) *"≥" means soluble, but saturation unknown. | ||
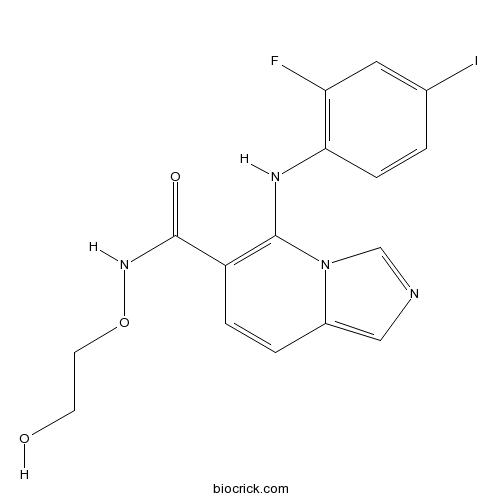
| Chemical Name | 5-(2-fluoro-4-iodoanilino)-N-(2-hydroxyethoxy)imidazo[1,5-a]pyridine-6-carboxamide | ||
| SMILES | C1=CC(=C(C=C1I)F)NC2=C(C=CC3=CN=CN32)C(=O)NOCCO | ||
| Standard InChIKey | RFWVETIZUQEJEF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14FIN4O3/c17-13-7-10(18)1-4-14(13)20-15-12(16(24)21-25-6-5-23)3-2-11-8-19-9-22(11)15/h1-4,7-9,20,23H,5-6H2,(H,21,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GDC-0623 is a potent and ATP-uncompetitive inhibitor of MEK1 with Ki value of 0.13 nM. | |||||
| Targets | MEK1 | |||||
| IC50 | 0.13 nM (Ki) | |||||

GDC-0623 Dilution Calculator

GDC-0623 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.192 mL | 10.9599 mL | 21.9197 mL | 43.8395 mL | 54.7993 mL |
| 5 mM | 0.4384 mL | 2.192 mL | 4.3839 mL | 8.7679 mL | 10.9599 mL |
| 10 mM | 0.2192 mL | 1.096 mL | 2.192 mL | 4.3839 mL | 5.4799 mL |
| 50 mM | 0.0438 mL | 0.2192 mL | 0.4384 mL | 0.8768 mL | 1.096 mL |
| 100 mM | 0.0219 mL | 0.1096 mL | 0.2192 mL | 0.4384 mL | 0.548 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GDC-0623 is a potent and ATP-uncompetitive inhibitor of MEK1 with Ki value of 0.13nM [1].
GDC-0623 is an allosteric MEK inhibitor and has efficacy against both mutant BRAF and mutant KRAS. In the cell viability assays, GDC-0623 inhibits BRAF (V600E) and KRAS (G13D) with EC50 values of 7nM and 42nM, respectively in A375 cells and HCT116 cells. Besides that, GDC-0623 shows similar efficacy in the two genotypes in a panel of BRAF and KRAS-mutant cancer cell lines. GDC-0623 is found to prevent MEK phosphorylation in cells, resulting in more effective inhibition of pERK. Furthermore, it is found that GDC-0623 blocks RAF activation through the effect on MEK. It induces dimerization of MEK with both BRAF and CRAF and stabilizes the RAF–MEK complex. In addition, GDC-0623 also suppresses RAF activation via inhibiting the formation of BRAF–CRAF heterodimer and the translocation of RAF in plasma membrane [1].
References:
[1] Hatzivassiliou G, Haling J R, Chen H, et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS-versus BRAF-driven cancers. Nature, 2013, 501(7466): 232-236.
- 4',5,6,7-Tetramethoxyflavone
Catalog No.:BCN8256
CAS No.:1168-42-9
- 2-(4-Hydroxyphenyl)-6-methyl-2,3-dihydro-4H-pyran-4-one
Catalog No.:BCN1610
CAS No.:1167483-18-2
- 9'''-Methyl salvianolate B
Catalog No.:BCN2923
CAS No.:1167424-32-9
- 9'-Methyl lithospermate B
Catalog No.:BCN2824
CAS No.:1167424-31-8
- Novaluron
Catalog No.:BCC5466
CAS No.:116714-46-6
- Glycyrrhisoflavone
Catalog No.:BCN2930
CAS No.:116709-70-7
- H-9 dihydrochloride
Catalog No.:BCC5656
CAS No.:116700-36-8
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- AZD7687
Catalog No.:BCC1394
CAS No.:1166827-44-6
- Mycophenolate mofetil hydrochloride
Catalog No.:BCC4117
CAS No.:116680-01-4
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- 5-Formamide-1-(2-formyloxyethl)pyrazole
Catalog No.:BCC8747
CAS No.:116856-18-9
- Fmoc-D-Ser-OH
Catalog No.:BCC3547
CAS No.:116861-26-8
- 20-Hydroxyaflavinine
Catalog No.:BCN7283
CAS No.:116865-08-8
- Monohydroxyisoaflavinine
Catalog No.:BCN7284
CAS No.:116865-09-9
- XL413
Catalog No.:BCC4241
CAS No.:1169558-38-6
- XL413 hydrochloride
Catalog No.:BCC4039
CAS No.:1169562-71-3
- INDY
Catalog No.:BCC6349
CAS No.:1169755-45-6
- Sculponeatin N
Catalog No.:BCN6044
CAS No.:1169805-98-4
- Sculponeatin O
Catalog No.:BCN6045
CAS No.:1169806-00-1
- Sculponeatic acid
Catalog No.:BCN6046
CAS No.:1169806-02-3
- Rubiadin
Catalog No.:BCN6047
CAS No.:117-02-2
- Dantron
Catalog No.:BCN6048
CAS No.:117-10-2
Elucidating the Mechanisms of Formation for Two Unusual Cytochrome P450-Mediated Fused Ring Metabolites of GDC-0623, a MAPK/ERK Kinase Inhibitor.[Pubmed:26438627]
Drug Metab Dispos. 2015 Dec;43(12):1929-33.
Two isomeric metabolites of GDC-0623 [5-((2-fluoro-4-iodophenyl)amino)-N-(2-hydroxyethoxy)imidazo[1,5-a]pyridine-6-car boxamide], a mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) kinase inhibitor, were identified in radiolabeled mass balance studies in rats and dogs (approximately 5% in excreta) and were also observed in human circulation (nonradiolabeled). Mass spectrometric data indicated that both metabolites had formed a new ring structure fused to the imidazopyridine core. Given their unusual structures, we conducted experiments to elucidate their chemical structures and understand the mechanisms for their formation. For the first metabolite, M14, a pyrazol-3-ol ring was generated by N-N bond formation between the aniline and hydroxamate. For the second metabolite, M13, an imidazol-2-one was generated by a Hofmann-type rearrangement that involved C-C bond cleavage and C-N bond formation. Both reactions were catalyzed by CYP2C9 and CYP2C19. M14 was generated directly from GDC-0623 and we speculate that its formation was via oxidative activation of the hydroxamic ester by cytochrome P450 (P450) and intramolecular nucleophilic displacement of the ester side chain. M13 (the rearranged metabolite) formed from the N-reduced hydroxamate (amide) and not from GDC-0623 directly. We propose for M13 that a P450-mediated reaction formed a cationic amide intermediate, which enabled the molecular rearrangement of the imidazopyridine core migrating from the amide carbon to the nitrogen and subsequent cyclization reaction. Each of these metabolic pathways constitutes a novel biotransformation mediated by P450 enzymes.


