Vemurafenib (PLX4032, RG7204)BRAF kinase inhibitor CAS# 918504-65-1 |
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- Dabrafenib (GSK2118436)
Catalog No.:BCC4393
CAS No.:1195765-45-7
- Dabrafenib Mesylate (GSK-2118436)
Catalog No.:BCC1513
CAS No.:1195768-06-9
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 918504-65-1 | SDF | Download SDF |
| PubChem ID | 42611257 | Appearance | Powder |
| Formula | C23H18ClF2N3O3S | M.Wt | 489.93 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | RG7204; R7204; RO5185426; PLX4032 | ||
| Solubility | DMSO : ≥ 100 mg/mL (204.11 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
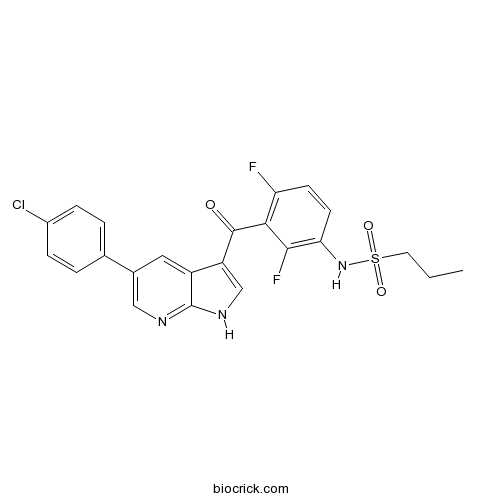
| Chemical Name | N-[3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluorophenyl]propane-1-sulfonamide | ||
| SMILES | CCCS(=O)(=O)NC1=C(C(=C(C=C1)F)C(=O)C2=CNC3=NC=C(C=C23)C4=CC=C(C=C4)Cl)F | ||
| Standard InChIKey | GPXBXXGIAQBQNI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H18ClF2N3O3S/c1-2-9-33(31,32)29-19-8-7-18(25)20(21(19)26)22(30)17-12-28-23-16(17)10-14(11-27-23)13-3-5-15(24)6-4-13/h3-8,10-12,29H,2,9H2,1H3,(H,27,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vemurafenib (PLX4032, RG7204) is a novel and potent inhibitor of B-RafV600E with IC50 of 31 nM. | |||||
| Targets | B-RafV600E | C-Raf | MAP4K5 (KHS1) | SRMS | ACK1 | FGR |
| IC50 | 31 nM | 48 nM | 51 nM | 18 nM | 19 nM | 63 nM |
| Cell experiment: | |
| Cell lines | MALME-3M melanoma cell lines |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 24 h; 10 μM |
| Applications | In melanoma cell lines, RG7204 was a potent inhibitor of proliferation in those expressing BRAFV600E but not BRAFWT. RG7204 also potently inhibited proliferation of melanoma cell lines expressing other codon 600 BRAF mutations (V600D, V600 K, and V600R). |
| Animal experiment: | |
| Animal models | Athymic nude mice |
| Dosage form | 100 mg/kg bid; oral taken. |
| Application | In mice bearing Colo829 tumor xenografts, RG7204 at 100 mg/kg bid for 21 days showed greatly improved antitumor activity compared both with vehicle (P = 0.001) at the end of the study on day 38 after the tumor cell implant. There was complete tumor regression in all 10 mice treated with RG7204 by the end of the study. Survival in the mice treated with RG7204 was significantly better than in those treated with vehicle (P = 0.0008). |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models[J]. Cancer research, 2010, 70(13): 5518-5527. | |

Vemurafenib (PLX4032, RG7204) Dilution Calculator

Vemurafenib (PLX4032, RG7204) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0411 mL | 10.2055 mL | 20.4111 mL | 40.8222 mL | 51.0277 mL |
| 5 mM | 0.4082 mL | 2.0411 mL | 4.0822 mL | 8.1644 mL | 10.2055 mL |
| 10 mM | 0.2041 mL | 1.0206 mL | 2.0411 mL | 4.0822 mL | 5.1028 mL |
| 50 mM | 0.0408 mL | 0.2041 mL | 0.4082 mL | 0.8164 mL | 1.0206 mL |
| 100 mM | 0.0204 mL | 0.1021 mL | 0.2041 mL | 0.4082 mL | 0.5103 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
Vemurafenib is a BRAF inhibitor that has been approved by FDA for the treatment of metastatic melanoma patients with a BRAF(V600E) mutation. Active efflux by P-gp and BCRP significantly restricted the brain distribution of vemurafenib at the blood-brain barrier, where active efflux by P-gp and BCRP restricted intracellular accumulation of vemurafenib and altered bidirectional net flux of vemurafenib.
Abstract
Vemurafenib is a mutated BRAF kinase inhibitor that showed response rates of >50% in metastatic melanoma patients with BRAF mutation. In a Phase III study, the treatment of vemurafenib in previously untreated patients led to over survival of 84%, response rates of 48% and prolonged progression-free survival with largely reduced risk of death and disease progression.
Abstract
Previous results of vemurafenib, a FDA-approved drug, were summarized to discuss its merits and limitations.
Abstract
The combined MAPK oncogene inhibition and metabolic modulation of AMPK is an effective treatment of melanoma cells due to a molecular linkage between the MAPK and the LKB1-AMPK pathways.
Abstract
Although the treatment of vemurafenib, a Braf inhibitor, in melanoma patients with Braf(V600E) mutations resulted in dramatic improvement with decreased risk of death and tumor progression, melanoma cells rapidly acquire vemurafenib resistance, which can be overcome by targeting Stat3-PAX3 signaling pathway.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Vemurafenib is an inhibitor of BRAF kinase. It inhibits BRAFV600E and also has inhibitory activity in vitro against several other kinds of kinases, including CRAF, ARAF and wild-type BRAF. Vemurafenib is a competitive small-molecule serine–threonine kinase inhibitor that functions by binding to the ATP-binding domain of mutant BRAF. Vemurafenib can also give rise to activation of downstream MEK by normal RAF homo- and heterodimers in non-BRAF mutated cells, which has been shown to be caused by transactivation of the nondrug-bound partner in BRAF to CRAF heterodimers and CRAF to CRAF homodimers.
Reference
Keith. T Flaherty, Uma Yasothan and Peter Kirkpatrick. Vemurafenib. Nature Reviews Drug Discovery. 2011; 10: 811 – 812.
Jason J. Luke, F. Stephen Hodi. Vemurafenib and BRAF Inhibition: A New Class of Treatment for Metastatic Melanoma. Clinical Cancer Research. 2012; 18: 9 – 14.
- 5,7,4'-Trimethoxyafzelechin
Catalog No.:BCN7933
CAS No.:918428-88-3
- Cefdinir
Catalog No.:BCC3747
CAS No.:91832-40-5
- UCL 2077
Catalog No.:BCC7446
CAS No.:918311-87-2
- MK-6892
Catalog No.:BCC1767
CAS No.:917910-45-3
- MK-2461
Catalog No.:BCC3816
CAS No.:917879-39-1
- CCMI
Catalog No.:BCC7788
CAS No.:917837-54-8
- PSB 0474
Catalog No.:BCC7459
CAS No.:917567-60-3
- Platycoside M3
Catalog No.:BCN3243
CAS No.:917482-69-0
- Platycoside M1
Catalog No.:BCN3238
CAS No.:917482-67-8
- Cyclo(Ile-Leu)
Catalog No.:BCN2434
CAS No.:91741-17-2
- Letermovir
Catalog No.:BCC1700
CAS No.:917389-32-3
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
- TH-302
Catalog No.:BCC1998
CAS No.:918633-87-1
- GPi 688
Catalog No.:BCC6091
CAS No.:918902-32-6
- 19-[(beta-D-glucopyranosyl)oxy]-19-oxo-ent-labda-8(17),13-dien-16,15-olide
Catalog No.:BCN1308
CAS No.:919120-78-8
- 1-Methoxyindole-3-carboxylic acid
Catalog No.:BCN3946
CAS No.:91913-76-7
- Atrial natriuretic factor (1-28) (human, porcine)
Catalog No.:BCC5839
CAS No.:91917-63-4
- Ro 15-4513
Catalog No.:BCC7230
CAS No.:91917-65-6
- Rubrisandrin A
Catalog No.:BCN3248
CAS No.:919289-30-8
- AZD1283
Catalog No.:BCC5370
CAS No.:919351-41-0
- Zatebradine hydrochloride
Catalog No.:BCC7286
CAS No.:91940-87-3
- Buergerinin B
Catalog No.:BCN4555
CAS No.:919769-83-8
Combination therapy with vemurafenib (PLX4032/RG7204) and metformin in melanoma cell lines with distinct driver mutations.[Pubmed:21609436]
J Transl Med. 2011 May 24;9:76.
BACKGROUND: A molecular linkage between the MAPK and the LKB1-AMPK energy sensor pathways suggests that combined MAPK oncogene inhibition and metabolic modulation of AMPK would be more effective than either manipulation alone in melanoma cell lines. MATERIALS AND METHODS: The combination of the BRAF inhibitor vemurafenib (formerly PLX4032) and metformin were tested against a panel of human melanoma cell lines with defined BRAF and NRAS mutations for effects on viability, cell cycle and apoptosis. Signaling molecules in the MAPK, PI3K-AKT and LKB1-AMPK pathways were studied by Western blot. RESULTS: Single agent metformin inhibited proliferation in 12 out of 19 cell lines irrespective of the BRAF mutation status, but in one NRASQ61K mutant cell line it powerfully stimulated cell growth. Synergistic anti-proliferative effects of the combination of metformin with vemurafenib were observed in 6 out of 11 BRAFV600E mutants, including highly synergistic effects in two BRAFV600E mutant melanoma cell lines. Antagonistic effects were noted in some cell lines, in particular in BRAFV600E mutant cell lines resistant to single agent vemurafenib. Seven out of 8 BRAF wild type cell lines showed marginally synergistic anti-proliferative effects with the combination, and one cell line had highly antagonistic effects with the combination. The differential effects were not dependent on the sensitivity to each drug alone, effects on cell cycle or signaling pathways. CONCLUSIONS: The combination of vemurafenib and metformin tended to have stronger anti-proliferative effects on BRAFV600E mutant cell lines. However, determinants of vemurafenib and metformin synergism or antagonism need to be understood with greater detail before any potential clinical utility of this combination.


