LetermovirNovel anti-CMV compound CAS# 917389-32-3 |
- Nevirapine
Catalog No.:BCC3820
CAS No.:129618-40-2
- Lamivudine
Catalog No.:BCC3801
CAS No.:134678-17-4
- Delavirdine
Catalog No.:BCC4300
CAS No.:136817-59-9
- Emtricitabine
Catalog No.:BCC3774
CAS No.:143491-57-0
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Delavirdine mesylate
Catalog No.:BCC4069
CAS No.:147221-93-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 917389-32-3 | SDF | Download SDF |
| PubChem ID | 45138674 | Appearance | Powder |
| Formula | C29H28F4N4O4 | M.Wt | 572.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AIC246 | ||
| Solubility | Soluble in DMSO | ||
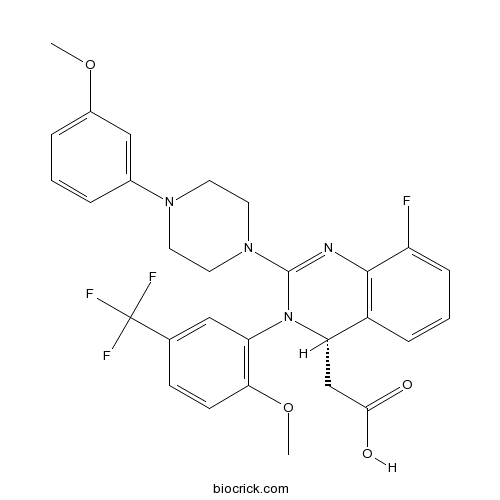
| Chemical Name | 2-[(4S)-8-fluoro-2-[4-(3-methoxyphenyl)piperazin-1-yl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-4H-quinazolin-4-yl]acetic acid | ||
| SMILES | COC1=C(C=C(C=C1)C(F)(F)F)N2C(C3=C(C(=CC=C3)F)N=C2N4CCN(CC4)C5=CC(=CC=C5)OC)CC(=O)O | ||
| Standard InChIKey | FWYSMLBETOMXAG-QHCPKHFHSA-N | ||
| Standard InChI | InChI=1S/C29H28F4N4O4/c1-40-20-6-3-5-19(16-20)35-11-13-36(14-12-35)28-34-27-21(7-4-8-22(27)30)23(17-26(38)39)37(28)24-15-18(29(31,32)33)9-10-25(24)41-2/h3-10,15-16,23H,11-14,17H2,1-2H3,(H,38,39)/t23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Letermovir is a novel inhibitor of CMV, which targets the viral terminase complex and remains active against virus resistant to DNA polymerase inhibitors.In Vitro:AIC246 has consistent antiviral efficacy, and there is remarkable selectivity of AIC246 for human cytomegaloviruses[1]. AD169 mutant strains and designated rAIC246-1 and rAIC246-2 are highly resistant to Letermovir (AIC246), with EC50s of 5.6 nM, 1.24 μM, 0.37 μM, respectively. Letermovir inhibits HCMV replication through a specific antiviral mechanism that involves the viral gene product UL56. Letermovir inhibits HCMV replication in cell culture by interfering with the proper cleavage/packaging of HCMV progeny DNA[2]. Letermovir inhibits the current gold standard GCV by more than 400-fold with respect to EC50s (mean, 4.5 nM versus 2 μM) and by more than 2,000-fold with respect to EC90 values (mean, 6.1 nM versus 14.5 μM)[3]. Letermovir in conbination with anti-HCMV drugs causes additive antiviral effects, but there is no interaction between letermovir and anti-HIV drugs[4].In Vivo:Letermovir (10-100 mg/kg/day, p.o.) leads to a dose-dependent reduction of the HCMV titer in transplanted cells compared to that of the placebo-treated control group using the mouse xenograft model[3]. References: | |||||

Letermovir Dilution Calculator

Letermovir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7466 mL | 8.7329 mL | 17.4657 mL | 34.9314 mL | 43.6643 mL |
| 5 mM | 0.3493 mL | 1.7466 mL | 3.4931 mL | 6.9863 mL | 8.7329 mL |
| 10 mM | 0.1747 mL | 0.8733 mL | 1.7466 mL | 3.4931 mL | 4.3664 mL |
| 50 mM | 0.0349 mL | 0.1747 mL | 0.3493 mL | 0.6986 mL | 0.8733 mL |
| 100 mM | 0.0175 mL | 0.0873 mL | 0.1747 mL | 0.3493 mL | 0.4366 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AIC246, also known as letermovir, is a novel anti-CMV compound with IC50 value of 5.1 ± 1.2 nM. It targets the pUL56 (amino acid 230-370) subunit of the viral terminase complex [1].
The subunit pUL56 is a component of the terminase complex which is responsible for packaging unit length DNA into assembling virions.
AIC246 has a novel mode of action targets the enzyme UL56 terminase and keep active to other drug-resistant virus. The anti-HCMV activity of AIC246 was evaluated in vitro by using different HCMV laboratory strains, GCV-resistant viruses. The result showed that the inhibitory potentcy of AIC246 surpasses the current gold standard GCV by more than 400-fold with respect to EC50s (mean, ∼4.5 nM versus ∼2 μM) and by more than 2,000-fold with respect to EC90 values (mean, ∼6.1 nM versus ∼14.5 μM). In the CPE-RA strains, the EC50 values of AIC 246 ranged from 1.8 nM to 6.1 nM [2].
In mouse model with HCMV subcutaneous xenograft, oral administration of AIC246 caused significant a dose-dependent reduction of the HCMV titer. 30 mg/kg/d AIC246 for 9 days induced PFU reduction with maximum efficiency, compared with the gold standard GCV at the ED50 and ED90 level [2].
References:
[1].Verghese PS, Schleiss MR. Letermovir Treatment of Human Cytomegalovirus Infection Anti-infective Agent. Drugs Future. 2013, 38(5):291-298.
[2]. Lischka P1, Hewlett G, Wunberg T, et al.In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246.Antimicrob Agents Chemother. 2010, 54(3):1290-1297.
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
- Bromfenac Sodium
Catalog No.:BCC4641
CAS No.:91714-93-1
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- 2-[(Acetylthio)methyl]-phenylpropionic acid
Catalog No.:BCC8507
CAS No.:91702-98-6
- Enniatin B
Catalog No.:BCN4774
CAS No.:917-13-5
- 5,7,4'-Trihydroxy-8-methylflavanone
Catalog No.:BCN2844
CAS No.:916917-28-7
- TC-I 15
Catalog No.:BCC6216
CAS No.:916734-43-5
- Clematiunicinoside E
Catalog No.:BCN7809
CAS No.:916649-92-8
- Clematomandshurica saponin B
Catalog No.:BCN7810
CAS No.:916649-91-7
- Coriatin
Catalog No.:BCN4457
CAS No.:91653-75-7
- Zingiberen newsaponin
Catalog No.:BCN2942
CAS No.:91653-50-8
- Senkyunolide C
Catalog No.:BCC9141
CAS No.:91652-78-7
- Cyclo(Ile-Leu)
Catalog No.:BCN2434
CAS No.:91741-17-2
- Platycoside M1
Catalog No.:BCN3238
CAS No.:917482-67-8
- Platycoside M3
Catalog No.:BCN3243
CAS No.:917482-69-0
- PSB 0474
Catalog No.:BCC7459
CAS No.:917567-60-3
- CCMI
Catalog No.:BCC7788
CAS No.:917837-54-8
- MK-2461
Catalog No.:BCC3816
CAS No.:917879-39-1
- MK-6892
Catalog No.:BCC1767
CAS No.:917910-45-3
- UCL 2077
Catalog No.:BCC7446
CAS No.:918311-87-2
- Cefdinir
Catalog No.:BCC3747
CAS No.:91832-40-5
- 5,7,4'-Trimethoxyafzelechin
Catalog No.:BCN7933
CAS No.:918428-88-3
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
Letermovir for the management of cytomegalovirus infection.[Pubmed:27998189]
Expert Opin Investig Drugs. 2017 Feb;26(2):235-241.
INTRODUCTION: Cytomegalovirus (CMV) is a major cause of morbidity and mortality in immunocompromised patients. Available antivirals are fraught with adverse effects and risk for the development of CMV resistance. Letermovir is a novel antiviral in the late stages of drug development for the treatment and prevention of CMV. Areas covered: A MEDLINE search of the MeSH terms 'Letermovir,' 'cytomegalovirus,' 'hematopoietic stem cell transplant,' and 'solid organ transplant,' was last conducted on 15 August 2016. Articles were selected on the basis of their contribution to current knowledge about Letermovir. Expert opinion: Letermovir's mechanism of action, pharmacokinetic and pharmacodynamic profile, and favorable efficacy and safety make it an attractive option for both the prevention and treatment of CMV in immunocompromised patients. The lack of cross-resistance with other antivirals and the absence of myelosuppression are two prominent characteristics of Letermovir that could support broad use of this product following FDA-approval. One major limitation is its lack of activity against other herpesviruses, which are commonly seen in immunocompromised hosts. We believe that with additional clinical efficacy data, this medication could emerge as a primary option for the prevention and treatment of CMV in the immunocompromised patient population.
Impact of glycoprotein B genotype and naturally occurring ORF UL56 polymorphisms upon susceptibility of clinical human cytomegalovirus isolates to letermovir.[Pubmed:27345658]
Antiviral Res. 2016 Aug;132:204-9.
Letermovir is a novel anti-HCMV drug in Phase III development that targets the UL56 subunit of the viral terminase complex. In immunocompromised patients four major glycoprotein B (gB) subtypes are known and may influence pathogenesis and thus disease outcomes. Using a panel of 74 Letermovir-naive, low-passage, clinical HCMV isolates, we examined the potential impact of i) gB genotype and ii) naturally occurring UL56 sequence variations upon susceptibility to Letermovir. Our data show that Letermovir's potency is independent of gB subtype and show that naturally-occurring Letermovir-resistance is rare or possibly absent.
HPLC Determination of Enantiomeric Purity of Letermovir Based on CHIRALPAK AD.[Pubmed:27048643]
J Chromatogr Sci. 2016 Apr 4. pii: bmw042.
A precise and sensitive LC method was developed and further validated for the determination of enantiomeric purity of {(4S)-8-Fluoro-2-[4-(3-methoxyphenyl)-1-piperazinyl]-3-[2-methoxy-5-(trifluoromet hyl)phenyl]-3,4-dihydro-4-quinazolinyl} acetic acid (Letermovir). Baseline separation with a resolution >2.8 was accomplished within 10 min using a CHIRALPAK AD (250 mm x 4.6 mm; particle size 5 mum) column, withn-hexane/2-propanol (80:20v/v) as mobile phase at a flow rate of 1 mL min(-1) The eluted analytes were monitored by UV detection at 260 nm. The effects of mobile phase composition and temperature on enantiomeric selectivity as well as resolution of enantiomers were thoroughly investigated. The calibration curves were plotted within the concentration range between 0.003 and 1 mg mL(-1)(n= 14), and the recoveries between 98.24 and 101.43% were obtained, with relative standard deviation <1.29%. The limit of detection (LOD) and limit of quantitation (LOQ) for Letermovir were 0.96 and 3.15 mug mL(-1); those for its enantiomer were 1.01 and 3.39 mug mL(-1), respectively. The developed method was demonstrated to be accurate, robust and sensitive for the determination of enantiomeric purity of Letermovir, especially for the analysis of bulk samples.
Pharmacokinetics and safety of letermovir, a novel anti-human cytomegalovirus drug, in patients with renal impairment.[Pubmed:28345163]
Br J Clin Pharmacol. 2017 Sep;83(9):1944-1953.
AIMS: Human cytomegalovirus remains a significant issue for immunocompromised patients and existing viral polymerase targeting therapies are associated with significant toxicity. Accordingly, the viral terminase complex inhibitor, Letermovir, is in development. We assessed Letermovir pharmacokinetics in renal impairment. METHODS: This was a Phase 1, open-label, nonrandomised trial. Estimated glomerular filtration rate based on the Modification of Diet Renal Disease equation was used to create three groups of eight subjects: healthy function (estimated glomerular filtration rate >/= 90 ml min(-1) 1.73m(-2) ), moderate (30-59 ml min(-1) 1.73m(-2) ) and severe (<30 ml min(-1) 1.73m(-2) ) impairment. Oral Letermovir 120 mg was dosed once-daily for 8 days and blood collected for pharmacokinetic analyses. RESULTS: All 24 subjects enrolled completed the trial. Moderate and severe renal impairment increased mean unbound Letermovir fractions by 11% and 26%, respectively, vs. healthy subjects. Exposure (AUCtau,ss and Css,max ) was increased with renal impairment [least square mean ratios (90% confidence intervals) total Letermovir vs. healthy subjects, AUCtau,ss 192% (143-258%) and 142% (83-243%) for moderate and severe impairment, respectively; Css,max 125% (87-182%) and 106% (75-151%), respectively]. Clearance was decreased vs. healthy subjects. Correlation analyses indicated a correlation between decreasing renal function and increased unbound Letermovir concentration (R(2) = 0.5076, P < 0.0001). Correlations were identified between decreased clearance with both decreased renal function (R(2) = 0.0662, P = 0.2249 and R(2) = 0.1861, P = 0.0353 total and unbound clearance, respectively) and increased age (R(2) = 0.3548, P = 0.0021 and R(2) = 0.3166, P = 0.0042 total and unbound clearance, respectively). Multiple-dose Letermovir 120 mg was well tolerated across groups. CONCLUSIONS: Renal impairment increased exposure to Letermovir, although age was a confounding factor.


