NevirapineCAS# 129618-40-2 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Didanosine
Catalog No.:BCC3763
CAS No.:69655-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 129618-40-2 | SDF | Download SDF |
| PubChem ID | 4463 | Appearance | Powder |
| Formula | C15H14N4O | M.Wt | 266.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BI-RG 587; NSC 641530; NVP | ||
| Solubility | DMSO : 14.29 mg/mL (53.66 mM; Need ultrasonic) | ||
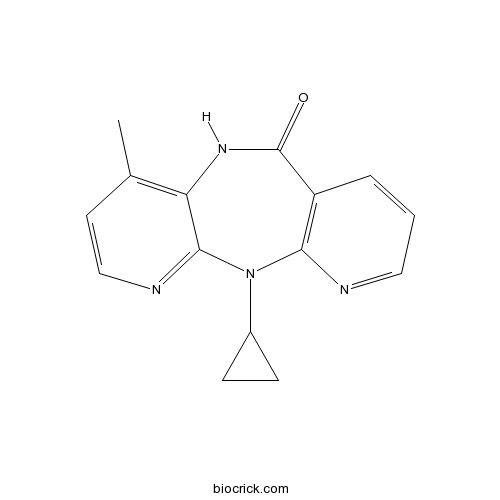
| Chemical Name | 11-cyclopropyl-4-methyl-5H-dipyrido[2,3-e:2',3'-f][1,4]diazepin-6-one | ||
| SMILES | CC1=C2C(=NC=C1)N(C3=C(C=CC=N3)C(=O)N2)C4CC4 | ||
| Standard InChIKey | NQDJXKOVJZTUJA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nevirapine is a non-nucleoside inhibitor of HIV-1 reverse transcriptase used to treat and prevent HIV/AIDS; with a Ki of 270 μM.In Vitro:Nevirapine itself is an inhibitor of only CYP3A4 at concentrations that are well above those of therapeutic relevance (Ki=270 μM)[1]. Nevirapine has been used as a re-differentiation agent to treat cancers in several human cancer models. At all doses (100, 200, 350, 500 μM) tested, nevirapine significantly inhibits cell proliferation after 48 h treatment. At high dose (500 μM), nevirapine significantly increases the percentage of apoptotic cells compared with control[2]. Nevirapine is a potent and selective inhibitor (IC50=10-100 nM) of the replication of a wide variety of HIV-1 strains in several cellular assays[3].In Vivo:Nevirapine is available for use in combination with nucleoside HIV-1 reverse transcriptase inhibitors (e.g., zidovudine, didanosine, etc.). Nevirapine has received FDA approval for use in combination with HIV-1 protease inhibitors (e.g., saquinavir, ritonavir, indinavir, etc.). In humans, nevirapine is eliminated primarily in the urine as glucuronide conjugates of 2-, 3-, 8-, and 12-hydroxynevirapine[1]. Nevirapine is completely absorbed in both sexes of mouse, rat, rabbit, monkey, and chimpanzee. Nevirapine is extensively metabolized in both sexes of all animal species studied[4]. Nevirapine (9 mg/kg, 18 mg/kg and 36 mg/kg) shows significant reduction in ulcer severity score and ulcer index as compared to the control[5] References: | |||||

Nevirapine Dilution Calculator

Nevirapine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7552 mL | 18.7758 mL | 37.5516 mL | 75.1033 mL | 93.8791 mL |
| 5 mM | 0.751 mL | 3.7552 mL | 7.5103 mL | 15.0207 mL | 18.7758 mL |
| 10 mM | 0.3755 mL | 1.8776 mL | 3.7552 mL | 7.5103 mL | 9.3879 mL |
| 50 mM | 0.0751 mL | 0.3755 mL | 0.751 mL | 1.5021 mL | 1.8776 mL |
| 100 mM | 0.0376 mL | 0.1878 mL | 0.3755 mL | 0.751 mL | 0.9388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A potent (IC50=84nM) and selective non-nucleoside inhibitor of HIV-1 reverse transcriptase.
- Satraplatin
Catalog No.:BCC5356
CAS No.:129580-63-8
- Goniopypyrone
Catalog No.:BCN3957
CAS No.:129578-07-0
- Tolfenpyrad
Catalog No.:BCC8069
CAS No.:129558-76-5
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- 3,4-Diacetoxycinnamamide
Catalog No.:BCN6157
CAS No.:129488-34-2
- Fmoc-Asp-OtBu
Catalog No.:BCC3088
CAS No.:129460-09-9
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Boc-Cysteinol(pMeBzl)
Catalog No.:BCC3044
CAS No.:129397-85-9
- Fmoc-Phenylalaninol
Catalog No.:BCC2717
CAS No.:129397-83-7
- Euphorbia factor L9
Catalog No.:BCN3786
CAS No.:129393-28-8
- Physcion 8-O-rutinoside
Catalog No.:BCN7324
CAS No.:129393-21-1
- 11-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8430
CAS No.:129385-59-7
- GR 82334
Catalog No.:BCC5802
CAS No.:129623-01-4
- 3-Hydroxybisabola-1,10-dien-9-one
Catalog No.:BCN7325
CAS No.:129673-86-5
- 3,4-Dihydroxybisabola-1,10-diene
Catalog No.:BCN7326
CAS No.:129673-87-6
- ZD 7114 hydrochloride
Catalog No.:BCC6852
CAS No.:129689-28-7
- Dofequidar
Catalog No.:BCC4176
CAS No.:129716-58-1
- Aripiprazole
Catalog No.:BCC5034
CAS No.:129722-12-9
- 2',4'-Dihydroxy-3',6'-dimethoxychalcone
Catalog No.:BCN6158
CAS No.:129724-43-2
- 3-Hydroxylanost-9(11)-24-dien-26-oic acid
Catalog No.:BCN1586
CAS No.:129724-83-0
- Anemoside A3
Catalog No.:BCN2328
CAS No.:129724-84-1
- Anemoside B4
Catalog No.:BCN1276
CAS No.:129741-57-7
- ODM-201
Catalog No.:BCC3796
CAS No.:1297538-32-9
- MC 976
Catalog No.:BCC1734
CAS No.:129831-99-8
A novel voltammetric sensor for nevirapine, based on modified graphite electrode by MWCNs/poly(methylene blue)/gold nanoparticle.[Pubmed:28366640]
Anal Biochem. 2017 Jun 15;527:4-12.
In the present study, a graphite electrode (GE) modified by conductive film (containing functionalized multi-walled carbon nanotubes (f-MWCNTs), poly methylene blue p(MB) and gold nanoparticles (AuNPs)) was introduced for determination of Nevirapine (NVP) as an anti-HIV drug by applying the differential pulse anodic stripping voltammetry (DPASV) technique. Modification of the electrode was investigated by scanning electron microscopy (SEM) and impedance electrochemical spectroscopy (EIS). All electrochemical effective parameters on detection of NVP were optimized and the oxidation peak current of drug was used for its monitoring. The obtained results confirmed that the oxidation peak currents increased linearly by increasing in NVP concentrations in the range of 0.1-50 muM and a detection limit of 53 nM was achieved. The proposed sensor (AuNPs/p(MB)/f-MWCNTs/GE) was successfully applied for the determination of NVP in blood serum and pharmaceutical samples. It revealed the excellent stability, repeatability and reproducibility as well.
Effect of Moringa oleifera Lam. leaf powder on the pharmacokinetics of nevirapine in HIV-infected adults: a one sequence cross-over study.[Pubmed:28293270]
AIDS Res Ther. 2017 Mar 14;14:12.
BACKGROUND: Moringa oleifera Lam., an herb commonly consumed by HIV-infected people on antiretroviral therapy, inhibits cytochrome P450 3A4, 1A2 and 2D6 activity in vitro; and may alter the pharmacokinetics (PK) of antiretroviral drugs metabolized via the same pathways. However, in vitro drug interaction activity may not translate to a clinically significant effect. Therefore, the effect of moringa leaf powder on the PK of Nevirapine in HIV-infected people was investigated. METHODS: Adult patients at steady-state dosing with Nevirapine were admitted for 12-h intensive PK sampling following a 21-day herbal medicine washout. Blood sampling was repeated after 14 days of Nevirapine and moringa (1.85 g leaf powder/day) co-administration. Nevirapine plasma concentrations were determined by liquid chromatography-tandem mass spectrometry. To assess the effect of moringa on Nevirapine PK, the change in Nevirapine area under the plasma concentration-time curve (AUC) was determined. The mean difference in pre- and post-moringa Nevirapine, maximum concentration (Cmax) and concentration at 12 h (C12h) were also calculated. The PK parameters were compared by assessing the post/pre geometric mean ratios (GMRs) and associated 90% confidence intervals (CIs). RESULTS: Pharmacokinetics analyses were performed on the results from 11 participants for whom complete data were obtained. The post/pre GMRs and associated 90% CIs for Nevirapine were 1.07 (1.00-1.14) for the AUC; 1.06 (0.98-1.16) for Cmax and 1.03 (0.92-1.16) for C12h. CONCLUSION: Co-administration of Moringa oleifera Lam. leaf powder at the traditional dose did not significantly alter the steady-state PK of Nevirapine. Trial registration number NCT01410058 (ClinicalTrials.gov).
Persistence of HIV drug resistance among South African children given nevirapine to prevent mother-to-child-transmission.[Pubmed:28301421]
AIDS. 2017 May 15;31(8):1143-1148.
OBJECTIVES: We set out to examine the prevalence and persistence of mutations conferring high-level nonnucleoside reverse transcriptase (NNRTI)-resistance in a cohort of HIV-infected children who had failed prophylaxis to prevent mother-to-child-transmission (PMTCT). DESIGN: A prospective observational cohort study at the Pediatric HIV Clinic at Kalafong Provincial Tertiary Hospital in Pretoria, South Africa. METHODS: Children referred for initiation of antiretroviral therapy (ART) were enrolled from July 2010 through February 2013. HIV drug resistance testing was performed using the oligonucleotide ligation assay (OLA) on dried blood spots (DBS) collected at enrolment and monthly follow-up visits for 2 years. RESULTS: South African children who failed HIV-prophylaxis had a high prevalence of NNRTI-resistant HIV (46/88; 52%). Among children with NNRTI-resistance, the frequency of the predominant resistant variant in each child's HIV-quasispecies was high (median 96%) at study entry (median age 7.5 months), and in 26 out of 27 followed a median of 13 months persisted at a high frequency (median 89%). CONCLUSION: Our finding that infants who fail HIV-prophylaxis frequently have long-lived NNRTI-resistant HIV suggests that resistance will likely persist through 36 months of age, when children qualify for NNRTI-based ART. These children may benefit from HIV drug resistance testing to guide selection of their treatment.


