SatraplatinCAS# 129580-63-8 |
- Tropisetron Hydrochloride
Catalog No.:BCC4027
CAS No.:105826-92-4
- Pizotifen
Catalog No.:BCC4215
CAS No.:15574-96-6
- Eletriptan HBr
Catalog No.:BCC5039
CAS No.:177834-92-3
- Fluvoxamine
Catalog No.:BCC4214
CAS No.:54739-18-3
- Lorcaserin HCl
Catalog No.:BCC5041
CAS No.:846589-98-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 129580-63-8 | SDF | Download SDF |
| PubChem ID | 11540590 | Appearance | Powder |
| Formula | C10H22Cl2N2O4Pt | M.Wt | 500.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BMS182751; BMY45594; JM216 | ||
| Solubility | DMSO : ≥ 33 mg/mL (65.96 mM; DMSO can inactivate Satraplatin's activity) *"≥" means soluble, but saturation unknown. | ||
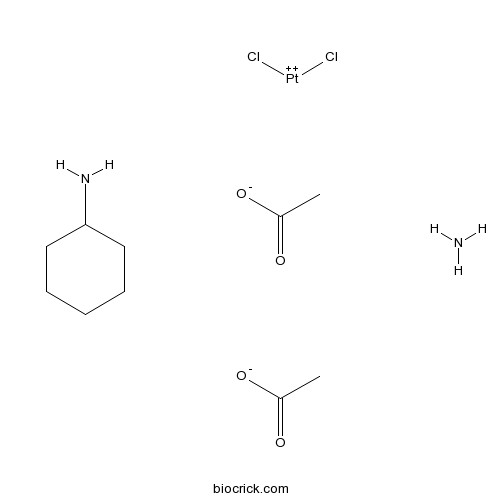
| Chemical Name | azane;cyclohexanamine;dichloroplatinum(2+);diacetate | ||
| SMILES | CC(=O)[O-].CC(=O)[O-].C1CCC(CC1)N.N.Cl[Pt+2]Cl | ||
| Standard InChIKey | CKNPWBAXEKSCRG-UHFFFAOYSA-J | ||
| Standard InChI | InChI=1S/C6H13N.2C2H4O2.2ClH.H3N.Pt/c7-6-4-2-1-3-5-6;2*1-2(3)4;;;;/h6H,1-5,7H2;2*1H3,(H,3,4);2*1H;1H3;/q;;;;;;+4/p-4 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Satraplatin Dilution Calculator

Satraplatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9989 mL | 9.9944 mL | 19.9888 mL | 39.9776 mL | 49.972 mL |
| 5 mM | 0.3998 mL | 1.9989 mL | 3.9978 mL | 7.9955 mL | 9.9944 mL |
| 10 mM | 0.1999 mL | 0.9994 mL | 1.9989 mL | 3.9978 mL | 4.9972 mL |
| 50 mM | 0.04 mL | 0.1999 mL | 0.3998 mL | 0.7996 mL | 0.9994 mL |
| 100 mM | 0.02 mL | 0.0999 mL | 0.1999 mL | 0.3998 mL | 0.4997 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Satraplatin is an alkylating agent, with potent antitumor effect.
In Vitro:Satraplatin has potent antitumor activity. Satraplatin combined with dichloroacetate (DCA) inhibits UMC-11 cells with an IC50 of 1.36 ± 0.11 μM[1]. Satraplatin also suppresses CDDP-resistant (KB-R) cells (IC50, 7.04 μM), and causes G2/M arrest in KB-R cells[2].
References:
[1]. Fiebiger W, et al. In vitro cytotoxicity of novel platinum-based drugs and dichloroacetate against lung carcinoid cell lines. Clin Transl Oncol. 2011 Jan;13(1):43-9.
[2]. Yamano Y, et al. Antitumor activity of satraplatin in cisplatin-resistant oral squamous cell carcinoma cells. Head Neck. 2011 Mar;33(3):309-17.
- Goniopypyrone
Catalog No.:BCN3957
CAS No.:129578-07-0
- Tolfenpyrad
Catalog No.:BCC8069
CAS No.:129558-76-5
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- 3,4-Diacetoxycinnamamide
Catalog No.:BCN6157
CAS No.:129488-34-2
- Fmoc-Asp-OtBu
Catalog No.:BCC3088
CAS No.:129460-09-9
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Boc-Cysteinol(pMeBzl)
Catalog No.:BCC3044
CAS No.:129397-85-9
- Fmoc-Phenylalaninol
Catalog No.:BCC2717
CAS No.:129397-83-7
- Euphorbia factor L9
Catalog No.:BCN3786
CAS No.:129393-28-8
- Physcion 8-O-rutinoside
Catalog No.:BCN7324
CAS No.:129393-21-1
- 11-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8430
CAS No.:129385-59-7
- O-Geranylconiferyl alcohol
Catalog No.:BCN4747
CAS No.:129350-09-0
- Nevirapine
Catalog No.:BCC3820
CAS No.:129618-40-2
- GR 82334
Catalog No.:BCC5802
CAS No.:129623-01-4
- 3-Hydroxybisabola-1,10-dien-9-one
Catalog No.:BCN7325
CAS No.:129673-86-5
- 3,4-Dihydroxybisabola-1,10-diene
Catalog No.:BCN7326
CAS No.:129673-87-6
- ZD 7114 hydrochloride
Catalog No.:BCC6852
CAS No.:129689-28-7
- Dofequidar
Catalog No.:BCC4176
CAS No.:129716-58-1
- Aripiprazole
Catalog No.:BCC5034
CAS No.:129722-12-9
- 2',4'-Dihydroxy-3',6'-dimethoxychalcone
Catalog No.:BCN6158
CAS No.:129724-43-2
- 3-Hydroxylanost-9(11)-24-dien-26-oic acid
Catalog No.:BCN1586
CAS No.:129724-83-0
- Anemoside A3
Catalog No.:BCN2328
CAS No.:129724-84-1
- Anemoside B4
Catalog No.:BCN1276
CAS No.:129741-57-7
- ODM-201
Catalog No.:BCC3796
CAS No.:1297538-32-9
Improvements in the Synthesis and Understanding of the Iodo-bridged Intermediate en Route to the Pt(IV) Prodrug Satraplatin.[Pubmed:25435590]
Inorganica Chim Acta. 2015 Jan 1;424:254-259.
Mixed amine/ammine motifs are important features in newer generation platinum anticancer agents, including the Pt(IV) prodrug Satraplatin. One synthetic route that can be used to access platinum molecules with such structures exploits the trans effect during NH3-mediated cleavage of iodo-bridged platinum(II) dimers of the form [Pt(Am)I(mu-I)]2, where Am is an amine. A clear picture of the nature of these dimers that is consistent with the reactivity they exhibit has remained elusive. Moreover, technical aspects of this chemistry have impeded its more widespread use. We present here an improved strategy that permits isolation and use of [Pt(Am)I(mu-I)]2, where Am is cyclohexylamine, within minutes as opposed to weeks, as previously reported. A detailed spectroscopic, crystallographic, and chromatographic investigation of this intermediate in the synthesis of Satraplatin is also presented with a discussion of the ability of both cis and trans isomers of the dimer to produce exclusively cis-[Pt(NH2C6H11)(NH3)I2] upon treatment with NH3.
Phase 1 trial and pharmacokinetic study of the oral platinum analog satraplatin in children and young adults with refractory solid tumors including brain tumors.[Pubmed:25556988]
Pediatr Blood Cancer. 2015 Apr;62(4):603-10.
BACKGROUND: Based on pre-clinical and clinical activity in adult refractory tumors, and absence of significant neuro-, nephro-, or oto-toxicity, we conducted a pediatric phase 1 trial to determine the toxicities, maximum tolerated dose (MTD), and pharmacokinetics of Satraplatin, an oral platinum analogue, in children and young adults with refractory solid tumors. PROCEDURE: Satraplatin was administered orally once daily on days 1-5 of a 28-day cycle at dose level (DL) 1 (60 mg/m(2) /dose), and DL2 (80 mg/m(2) /dose). Toxicities, responses, Satraplatin pharmacokinetics, and pharmacogenomic expression of specific DNA repair genes were evaluated. RESULTS: Nine patients received 1-15 cycles (median = 2). The MTD was exceeded at DL2 with delayed prolonged myelosuppression as dose-limiting toxicity (DLT) in 2/4 patients. At DL1, 0/5 patients had DLTs. Common non-DLTs included myelosuppression, gastrointestinal toxicities, fatigue, headache, liver enzyme elevation, and electrolyte abnormalities. No significant neuro-, nephro-, or oto-toxicity was observed. No objective responses were observed but 2 patients experienced prolonged disease stabilization (---6-15 cycles). Satraplatin exposure (day 1 plasma ultrafiltrate area under the curve) was similar at DL1 and DL2. A strong correlation between estimated creatinine clearance and Satraplatin pharmacokinetic parameters (clearance, area under the curve, and peak concentration) was observed. CONCLUSIONS: The MTD of oral Satraplatin in children with solid tumors was 60 mg/m(2) /dose daily x5 days every 28 days, which is lower than the adult recommended dose of 80-120 mg/m(2) /dose. The toxicity profile was similar to adults and delayed myelosuppression was the DLT. No significant neuro-, nephro- or oto-toxicities were observed.
Electrochemical Detection of Platinum(IV) Prodrug Satraplatin in Serum.[Pubmed:26465061]
Anal Chem. 2015 Nov 3;87(21):11092-7.
We report the design and fabrication of a reagentless and reusable electrochemical sensor for detection of Satraplatin (SAT), a platinum(IV) prodrug. The detection strategy is based on the electrocatalytic reaction between the Pt(IV) center of SAT and surface-immobilized methylene blue. We systematically evaluated the effect of passivating diluent chain length on the overall sensor performance. Our results show that the use of a shorter diluent like 2-mercaptoethanol is more advantageous than using a longer and more passivating diluent such as 6-mercapto-1-hexanol. Independent of the use of cyclic voltammetry or chronoamperometry as the sensor interrogation technique, all three sensors, each passivated with a different alkanethiol diluent, have been demonstrated to be sensitive; the limit of detection is in the range of 1-10 muM. They are also highly specific and do not respond to Pt(II) drugs such as cisplatin and carboplatin. More importantly, they are selective enough to be employed directly in 50% serum. This sensing strategy has potential applications in clinical pharmacokinetics studies.
Modulation of signaling enhances the efficacy of the combination of satraplatin and erlotinib.[Pubmed:25382189]
Curr Drug Targets. 2014;15(14):1312-21.
UNLABELLED: The active metabolite (JM118) of the oral platinum analog Satraplatin (JM216) was investigated for potential synergism with erlotinib, an epidermal growth factor receptor (EGFR) inhibitor. JM118 sensitivity of 7 cancer cell lines (ovarian: 2008, A2780; colon: Lovo92, WiDr; lung: A549, SW1573; epidermoid: A431), was enhanced most pronounced when JM118 preceded erlotinib, which was associated with increased formation of DNA-platinum adducts. The combination increased G2/M phase accumulation and enhanced apoptosis. JM118 increased the phosphorylation of the cell cycle proteins CDK2 and CHK1 after 24 hr exposure. JM118/erlotinib enhanced Erk and Akt phosphorylation after 2 hr. JM118 significantly decreased the phosphorylation of PTEN, VEGFR, EPHA1, ERBB4, FGF-R, andSTAT3 by 20 (PTEN) to >90% (STAT3). CONCLUSION: Erlotinib enhanced the effects of JM118, even in cells with mutations in Ras. The mechanism of synergy involved a combination of effects on platinum-DNA adduct formation, cell cycle distribution and signaling.


