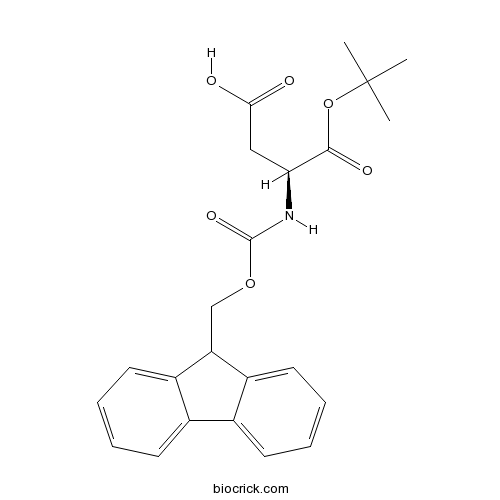
Fmoc-Asp-OtBuCAS# 129460-09-9 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 129460-09-9 | SDF | Download SDF |
| PubChem ID | 7017910 | Appearance | Powder |
| Formula | C23H25NO6 | M.Wt | 411.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | (3S)-3-(9H-fluoren-9-ylmethoxycarbonylamino)-4-[(2-methylpropan-2-yl)oxy]-4-oxobutanoic acid | ||
| SMILES | CC(C)(C)OC(=O)C(CC(=O)O)NC(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13 | ||
| Standard InChIKey | VZXQYACYLGRQJU-IBGZPJMESA-N | ||
| Standard InChI | InChI=1S/C23H25NO6/c1-23(2,3)30-21(27)19(12-20(25)26)24-22(28)29-13-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,18-19H,12-13H2,1-3H3,(H,24,28)(H,25,26)/t19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Asp-OtBu Dilution Calculator

Fmoc-Asp-OtBu Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4301 mL | 12.1507 mL | 24.3013 mL | 48.6027 mL | 60.7533 mL |
| 5 mM | 0.486 mL | 2.4301 mL | 4.8603 mL | 9.7205 mL | 12.1507 mL |
| 10 mM | 0.243 mL | 1.2151 mL | 2.4301 mL | 4.8603 mL | 6.0753 mL |
| 50 mM | 0.0486 mL | 0.243 mL | 0.486 mL | 0.9721 mL | 1.2151 mL |
| 100 mM | 0.0243 mL | 0.1215 mL | 0.243 mL | 0.486 mL | 0.6075 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Asp-OtBu
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Boc-Cysteinol(pMeBzl)
Catalog No.:BCC3044
CAS No.:129397-85-9
- Fmoc-Phenylalaninol
Catalog No.:BCC2717
CAS No.:129397-83-7
- Euphorbia factor L9
Catalog No.:BCN3786
CAS No.:129393-28-8
- Physcion 8-O-rutinoside
Catalog No.:BCN7324
CAS No.:129393-21-1
- 11-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8430
CAS No.:129385-59-7
- O-Geranylconiferyl alcohol
Catalog No.:BCN4747
CAS No.:129350-09-0
- Alendronate sodium
Catalog No.:BCC3719
CAS No.:129318-43-0
- Licorisoflavan A
Catalog No.:BCN6662
CAS No.:129314-37-0
- Isoangustone A
Catalog No.:BCN6819
CAS No.:129280-34-8
- Semilicoisoflavone B
Catalog No.:BCN2931
CAS No.:129280-33-7
- PF-2545920
Catalog No.:BCC2279
CAS No.:1292799-56-4
- 3,4-Diacetoxycinnamamide
Catalog No.:BCN6157
CAS No.:129488-34-2
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Tolfenpyrad
Catalog No.:BCC8069
CAS No.:129558-76-5
- Goniopypyrone
Catalog No.:BCN3957
CAS No.:129578-07-0
- Satraplatin
Catalog No.:BCC5356
CAS No.:129580-63-8
- Nevirapine
Catalog No.:BCC3820
CAS No.:129618-40-2
- GR 82334
Catalog No.:BCC5802
CAS No.:129623-01-4
- 3-Hydroxybisabola-1,10-dien-9-one
Catalog No.:BCN7325
CAS No.:129673-86-5
- 3,4-Dihydroxybisabola-1,10-diene
Catalog No.:BCN7326
CAS No.:129673-87-6
- ZD 7114 hydrochloride
Catalog No.:BCC6852
CAS No.:129689-28-7
- Dofequidar
Catalog No.:BCC4176
CAS No.:129716-58-1
- Aripiprazole
Catalog No.:BCC5034
CAS No.:129722-12-9
New t-butyl based aspartate protecting groups preventing aspartimide formation in Fmoc SPPS.[Pubmed:26077723]
J Pept Sci. 2015 Aug;21(8):680-7.
Obtaining homogenous aspartyl-containing peptides via Fmoc/tBu chemistry is often an insurmountable obstacle. A generic solution for this issue utilising an optimised side-chain protection strategy that minimises aspartimide formation would therefore be most desirable. To this end, we developed the following new derivatives: Fmoc-Asp(OEpe)-OH (Epe = 3-ethyl-3-pentyl), Fmoc-Asp(OPhp)-OH (Php = 4-n-propyl-4-heptyl) and Fmoc-Asp(OBno)-OH (Bno = 5-n-butyl-5-nonyl). We have compared their effectiveness against that of Fmoc-Asp(OtBu)-OH and Fmoc-Asp(OMpe)-OH in the well-established scorpion toxin II model peptide variants H-Val-Lys-Asp-Asn/Arg-Tyr-Ile-OH by treatments of the peptidyl resins with the Fmoc removal reagents containing piperidine and DBU at both room and elevated temperatures. The new derivatives proved to be extremely effective in minimising aspartimide by-products in each application.
Parallel high-throughput accurate mass measurement using a nine-channel multiplexed electrospray liquid chromatography ultraviolet time-of-flight mass spectrometry system.[Pubmed:12820207]
Rapid Commun Mass Spectrom. 2003;17(13):1425-32.
A nine-channel multiplexed electrospray (MUX) liquid chromatography ultraviolet time-of-flight mass spectrometry (LC/UV/TOFMS) system has been used to simultaneously measure accurate masses of eluting components from eight parallel gradient LC columns. Accuracies better than 5 and 10 ppm were achieved for 50 and 80% of samples, respectively, from a single batch analysis of ten plates (960 samples) of a Fmoc-Asp(OtBu)-OH and reserpine mixture. Combinatorial library compounds were analyzed using this parallel high-throughput system in both positive and negative modes to rigorously verify expected products and identify side products. A mass accuracy of 10 ppm root mean square (RMS) is routinely obtained for combinatorial library samples from this high-throughput accurate mass LC/MS system followed by automated data processing. This mass accuracy is critical in revealing combinatorial synthesis problems that would be missed by unit mass measurement.
Synthesis of prothymosin alpha deduced from nucleotide sequence of the murine cDNA and its effect on the impaired T lymphocytes of uremic patients.[Pubmed:8292970]
Biotechnol Ther. 1993;4(3-4):213-20.
The complete murine prothymosin alpha molecule (110 residues) except for the N-terminal methionine deduced from the cloned cDNA has been synthesized by a solid-phase method. Peptide synthesis was performed manually by the stepwise solid-phase method using the base-labile Fmoc group for protecting the alpha-amino group. The peptide was assembled on a p-alkoxybenzyl alcohol resin. After the last coupling step, the Fmoc group was removed with 50% piperidine in DMF. The peptide resin was treated with thioanisole-o-cresol in TFA, and then purified by gel filtration, ion-exchange column chromatography and high-performance liquid chromatography. A 2.9-mg sample of a highly purified peptide was finally obtained. The overall yield of the synthesis was less than 1%, based on the amino acid content of the starting Fmoc-Asp (OtBu)-resin. The synthetic peptide was found to have a restoring activity on low-E-rosette-forming lymphocytes after incubation of peripheral blood from uremic patients with the synthetic peptide. This peptide exhibited far stronger restoring effect than that of our synthetic thymosin alpha 1.
A 'conovenomic' analysis of the milked venom from the mollusk-hunting cone snail Conus textile--the pharmacological importance of post-translational modifications.[Pubmed:24055806]
Peptides. 2013 Nov;49:145-58.
Cone snail venoms provide a largely untapped source of novel peptide drug leads. To enhance the discovery phase, a detailed comparative proteomic analysis was undertaken on milked venom from the mollusk-hunting cone snail, Conus textile, from three different geographic locations (Hawai'i, American Samoa and Australia's Great Barrier Reef). A novel milked venom conopeptide rich in post-translational modifications was discovered, characterized and named alpha-conotoxin TxIC. We assign this conopeptide to the 4/7 alpha-conotoxin family based on the peptide's sequence homology and cDNA pre-propeptide alignment. Pharmacologically, alpha-conotoxin TxIC demonstrates minimal activity on human acetylcholine receptor models (100 muM, <5% inhibition), compared to its high paralytic potency in invertebrates, PD50 = 34.2 nMol kg(-1). The non-post-translationally modified form, [Pro](2,8)[Glu](16)alpha-conotoxin TxIC, demonstrates differential selectivity for the alpha3beta2 isoform of the nicotinic acetylcholine receptor with maximal inhibition of 96% and an observed IC50 of 5.4 +/- 0.5 muM. Interestingly its comparative PD50 (3.6 muMol kg(-1)) in invertebrates was ~100 fold more than that of the native peptide. Differentiating alpha-conotoxin TxIC from other alpha-conotoxins is the high degree of post-translational modification (44% of residues). This includes the incorporation of gamma-carboxyglutamic acid, two moieties of 4-trans hydroxyproline, two disulfide bond linkages, and C-terminal amidation. These findings expand upon the known chemical diversity of alpha-conotoxins and illustrate a potential driver of toxin phyla-selectivity within Conus.
On the use of N-dicyclopropylmethyl aspartyl-glycine synthone for backbone amide protection.[Pubmed:19924731]
J Pept Sci. 2010 Jan;16(1):65-70.
To prevent aspartimide formation and related side products in Asp-Xaa, particularly Asp-Gly-containing peptides, usually the 2-hydroxy-4-methoxybenzyl (Hmb) backbone amide protection is applied for peptide synthesis according to the Fmoc-protocols. In the present study, the usefulness of the recently proposed acid-labile dicyclopropylmethyl (Dcpm) protectant was analyzed. Despite the significant steric hindrance of this bulky group, N-terminal H-(Dcpm)Gly-peptides are quantitatively acylated by potent acylating agents, and alternatively the dipeptide Fmoc-Asp(OtBu)-(Dcpm)Gly-OH derivative can be used as a building block. In contrast to the Hmb group, Dcpm is inert toward acylations, but is readily removed in the acid deprotection and resin-cleavage step.


