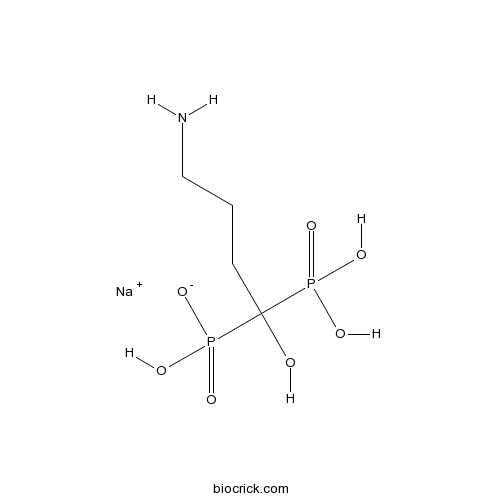
Alendronate sodiumOsteoclast-mediated bone resorption inhibitor CAS# 129318-43-0 |
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- L-165041
Catalog No.:BCC1687
CAS No.:79558-09-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 129318-43-0 | SDF | Download SDF |
| PubChem ID | 62958 | Appearance | Powder |
| Formula | C4H12NNaO7P2 | M.Wt | 271.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Alendronate sodium | ||
| Solubility | Soluble to 50 mM in water | ||
| Chemical Name | sodium;(4-amino-1-hydroxy-1-phosphonobutyl)-hydroxyphosphinate | ||
| SMILES | C(CC(O)(P(=O)(O)O)P(=O)(O)[O-])CN.[Na+] | ||
| Standard InChIKey | CAKRAHQRJGUPIG-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C4H13NO7P2.Na/c5-3-1-2-4(6,13(7,8)9)14(10,11)12;/h6H,1-3,5H2,(H2,7,8,9)(H2,10,11,12);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Osteoclast-mediated bone resorption inhibitor. Binds and blocks farnesyl diphosphate synthase (FPPS) in the HMG-CoA pathway (IC50 = 460 nM for recombinant human FPPS); causes macrophage apoptosis. Inhibits prenylation and sterol biosynthesis in purified osteoclasts. |

Alendronate sodium Dilution Calculator

Alendronate sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6889 mL | 18.4447 mL | 36.8895 mL | 73.779 mL | 92.2237 mL |
| 5 mM | 0.7378 mL | 3.6889 mL | 7.3779 mL | 14.7558 mL | 18.4447 mL |
| 10 mM | 0.3689 mL | 1.8445 mL | 3.6889 mL | 7.3779 mL | 9.2224 mL |
| 50 mM | 0.0738 mL | 0.3689 mL | 0.7378 mL | 1.4756 mL | 1.8445 mL |
| 100 mM | 0.0369 mL | 0.1844 mL | 0.3689 mL | 0.7378 mL | 0.9222 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Osteoclast-mediated bone resorption inhibitor. Binds and blocks farnesyl diphosphate synthase (FPPS) in the HMG-CoA pathway (IC50 = 460 nM for recombinant human FPPS); causes macrophage apoptosis. Inhibits prenylation and sterol biosynthesis in purified osteoclasts.
- Licorisoflavan A
Catalog No.:BCN6662
CAS No.:129314-37-0
- Isoangustone A
Catalog No.:BCN6819
CAS No.:129280-34-8
- Semilicoisoflavone B
Catalog No.:BCN2931
CAS No.:129280-33-7
- PF-2545920
Catalog No.:BCC2279
CAS No.:1292799-56-4
- Boscialin
Catalog No.:BCN7323
CAS No.:129277-03-8
- Pierreione B
Catalog No.:BCN6855
CAS No.:1292766-21-2
- Perospirone hydrochloride
Catalog No.:BCC9118
CAS No.:129273-38-7
- Hoechst 33258 analog 6
Catalog No.:BCC1628
CAS No.:129244-66-2
- IKKε-IN-1
Catalog No.:BCC5514
CAS No.:1292310-49-6
- 15-Dihydroepioxylubimin
Catalog No.:BCN4800
CAS No.:129214-59-1
- (2S,3S)-(-)-Glucodistylin
Catalog No.:BCN6156
CAS No.:129212-92-6
- Iberiotoxin
Catalog No.:BCC6932
CAS No.:129203-60-7
- O-Geranylconiferyl alcohol
Catalog No.:BCN4747
CAS No.:129350-09-0
- 11-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8430
CAS No.:129385-59-7
- Physcion 8-O-rutinoside
Catalog No.:BCN7324
CAS No.:129393-21-1
- Euphorbia factor L9
Catalog No.:BCN3786
CAS No.:129393-28-8
- Fmoc-Phenylalaninol
Catalog No.:BCC2717
CAS No.:129397-83-7
- Boc-Cysteinol(pMeBzl)
Catalog No.:BCC3044
CAS No.:129397-85-9
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Fmoc-Asp-OtBu
Catalog No.:BCC3088
CAS No.:129460-09-9
- 3,4-Diacetoxycinnamamide
Catalog No.:BCN6157
CAS No.:129488-34-2
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Tolfenpyrad
Catalog No.:BCC8069
CAS No.:129558-76-5
- Goniopypyrone
Catalog No.:BCN3957
CAS No.:129578-07-0
Short term sodium alendronate administration improves the peri-implant bone quality in osteoporotic animals.[Pubmed:28198975]
J Appl Oral Sci. 2017 Jan-Feb;25(1):42-52.
Objective: The aim of this study was to evaluate the bone repair process at the bone/implant interface of osteoporotic rats treated with sodium alendronate through the analysis of microtomography, real time polymerase chain reactions and immunohistochemistry (RUNX2 protein, bone sialoprotein (BSP), alkaline phosphatase, osteopontin and osteocalcin). Material and Methods: A total of 42 rats were used and divided in to the following experimental groups: CTL: control group (rats submitted to fictitious surgery and fed with a balanced diet), OST: osteoporosis group (rats submitted to a bilateral ovariectomy and fed with a low calcium diet) and ALE: alendronate group (rats submitted to a bilateral ovariectomy, fed with a low calcium diet and treated with sodium alendronate). A surface treated implant was installed in both tibial metaphyses of each rat. Euthanasia of the animals was conducted at 14 (immunhostochemistry) and 42 days (immunohistochemistry, micro CT and PCR). Data were subjected to statistical analysis with a 5% significance level. Results: Bone volume (BV) and total pore volume were higher for ALE group (P<0.05). Molecular data for RUNX2 and BSP proteins were significantly expressed in the ALE group (P<0.05), in comparison with the other groups. ALP expression was higher in the CTL group (P<0.05). The immunostaining for RUNX2 and osteopontin was positive in the osteoblastic lineage cells of neoformed bone for the CTL and ALE groups in both periods (14 and 42 days). Alkaline phosphatase presented a lower staining area in the OST group compared to the CTL in both periods and the ALE at 42 days. Conclusion: There was a decrease of osteocalcin precipitation at 42 days for the ALE and OST groups. Therefore, treatment with short-term sodium alendronate improved bone repair around the implants installed in the tibia of osteoporotic rats.
Pharmacokinetics of coadministration of levothyroxine sodium and alendronate sodium new effervescent formulation.[Pubmed:28204953]
Osteoporos Int. 2017 May;28(5):1745-1752.
No clinically important pharmacokinetic interference of alendronate occurred between a new effervescent formulation of alendronate and levothyroxine when coadministered. The combination does not materially affect levothyroxine absorption. INTRODUCTION: Concurrent treatment of osteoporosis with alendronate (Aln) and hypothyroidism with levothyroxine (LT4) may be problematic because both drugs are to be taken separately after fasting overnight. The primary objective was to assess pharmacokinetic interactions between a new effervescent formulation of Aln (Aln-NEF) and LT4. METHODS: A randomized, open-label, 3-way crossover study was conducted in 30 healthy adults (15 women). Subjects were dosed 3 times, separated by 35 days, after overnight fasts, with Aln-NEF alone (70 mg), LT4 alone (600 mug), or Aln-NEF and LT4 concurrently. Samples were analyzed for plasma Aln and serum LT4. Pharmacokinetic drug-drug interaction was assessed using 90% confidence intervals (CIs) for the test/reference ratio of the geometric means for area under the concentration-time curve from time zero to last measureable time point (AUC0-t ) and maximum concentration (C max). Results were compared to the default no-effect boundaries of 80 to 125% for the ratio Aln-NEF and LT4 concurrently/Aln-NEF alone and the ratio Aln-NEF and LT4 concurrently/LT4 alone. RESULTS: Geometric mean ratios (Aln-NEF with LT4/Aln-NEF alone) were 0.927 (90% CI 0.795-1.081) for AUC0-8 and 0.912 (90% CI 0.773-1.077) for C max, demonstrating LT4 does not appreciably affect the pharmacokinetics of Aln. Geometric mean ratios (LT4 with Aln-NEF/LT4 alone) were 1.049 (90% CI 0.983-1.119) for AUC0-48 and 1.075 (90% CI 1.006-1.148) for C max, demonstrating LT4 is bioequivalent between the 2 treatments. Coadministration of Aln-NEF and LT4 was well tolerated. CONCLUSIONS: There was no clinically important pharmacokinetic interference between the Aln-NEF formulation and LT4. Aln-NEF does not materially affect LT4 absorption.
A Comparative Study of Intravenous Injection Form and Oral Jelly Form of Alendronate Sodium Hydrate for Bone Mineral Disorder after Gastrectomy.[Pubmed:28214864]
Digestion. 2017;95(2):162-171.
BACKGROUND/AIMS: Osteoporosis is found to have high prevalence after gastrectomy and therefore, it is important to prevent this condition by means of effective medication, such as Alendronate sodium hydrate. METHODS: A total number of 48 gastric cancer patients diagnosed with osteoporosis after R0 gastrectomy was registered in this study between December 2013 and August 2014. Twenty-three patients received intravenous (i.v.) Alendronate sodium hydrate and 25 patients received the drug in an oral jelly form. Serological and urinary examinations related to bone metabolism and bone mineral density (BMD) were performed periodically and the results obtained from the 2 groups were compared. RESULTS: BMD increased, serum levels of bone-specific alkaline phosphatase and tartrate-resistant acid phosphatase-5b, and the urine level of urine N-terminal telopeptide decreased with time in both groups. However, the serum Ca level did not change. Two-way analysis of variance revealed no significant differences in these factors between the 2 groups. CONCLUSION: It is essential to prevent both forms of osteoporosis by using Alendronate sodium hydrate after gastrectomy for gastric cancer. A prospective, randomized, controlled trial in many patients following long duration should be conducted to clarify the benefits of i.v. Alendronate sodium hydrate.
Study on critical-sized ultra-high molecular weight polyethylene wear particles loaded with alendronate sodium: in vitro release and cell response.[Pubmed:28210968]
J Mater Sci Mater Med. 2017 Apr;28(4):56.
The aim of this study was to investigate the in vitro release and the effect of RAW 264.7 macrophages of critical-sized wear particles of ultra-high molecular weight polyethylene (UHMWPE) loaded with Alendronate sodium (ALN), one of the most effective drugs to treat osteoporosis in clinic. The critical-sized UHMWPE-ALN 0.5 wt.% wear particles were prepared by vacuum gradient filtration combined with Pluronic F-68. In vitro release of ALN from critical-sized UHMWPE-ALN wear particles was investigated in phosphate buffered saline (PBS) at 37 degrees C with a shaker. Cell morphology, proliferation, lactate dehydrogenase (LDH) leakage and secretions of cytokines were evaluated after co-cultured with critical-sized UHMWPE-ALN wear particles in vitro. Results showed that ALN released from critical-sized UHMWPE-ALN wear particles included burst release and slow release in vitro. Macrophages would be chemotaxis and aggregated around the critical-sized UHMWPE-ALN or UHMWPE wear particle, which was phagocytosed with time. The proliferation of macrophages co-cultured with critical-sized UHMWPE-ALN wear particles was significantly decreased compared with that of critical-sized UHMWPE group. Meanwhile, the critical-sized UHMWPE-ALN wear particles significantly induced the LDH leakage of macrophages, which indicated the cell death. The death of macrophages induced by ALN was one of pathways to inhibit their proliferation. The secretions of cytokines (interleukin-6 and tumor necrosis factor-alpha) in critical-sized UHMWPE-ALN group were significantly lower than those in critical-sized UHMWPE group due to the released ALN. The present results suggested that UHMWPE-ALN had the potential application in clinic to treat osteolysis induced by wear particles.
Lowering bone mineral affinity of bisphosphonates as a therapeutic strategy to optimize skeletal tumor growth inhibition in vivo.[Pubmed:18974139]
Cancer Res. 2008 Nov 1;68(21):8945-53.
Bisphosphonates bind avidly to bone mineral and are potent inhibitors of osteoclast-mediated bone destruction. They also exhibit antitumor activity in vitro. Here, we used a mouse model of human breast cancer bone metastasis to examine the effects of risedronate and NE-10790, a phosphonocarboxylate analogue of the bisphosphonate risedronate, on osteolysis and tumor growth. Osteolysis was measured by radiography and histomorphometry. Tumor burden was measured by fluorescence imaging and histomorphometry. NE-10790 had a 70-fold lower bone mineral affinity compared with risedronate. It was 7-fold and 8,800-fold less potent than risedronate at reducing, respectively, breast cancer cell viability in vitro and bone loss in ovariectomized animals. We next showed that risedronate given at a low dosage in animals bearing human B02-GFP breast tumors reduced osteolysis by inhibiting bone resorption, whereas therapy with higher doses also inhibited skeletal tumor burden. Conversely, therapy with NE-10790 substantially reduced skeletal tumor growth at a dosage that did not inhibit osteolysis, a higher dosage being able to also reduce bone destruction. The in vivo antitumor activity of NE-10790 was restricted to bone because it did not inhibit the growth of subcutaneous B02-GFP tumor xenografts nor the formation of B16-F10 melanoma lung metastases. Moreover, NE-10790, in combination with risedronate, reduced both osteolysis and skeletal tumor burden, whereas NE-10790 or risedronate alone only decreased either tumor burden or osteolysis, respectively. In conclusion, our study shows that decreasing the bone mineral affinity of bisphosphonates is an effective therapeutic strategy to inhibit skeletal tumor growth in vivo.
Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase.[Pubmed:10620343]
Arch Biochem Biophys. 2000 Jan 1;373(1):231-41.
Alendronate, a nitrogen-containing bisphosphonate, is a potent inhibitor of bone resorption used for the treatment and prevention of osteoporosis. Recent findings suggest that alendronate and other N-containing bisphosphonates inhibit the isoprenoid biosynthesis pathway and interfere with protein prenylation, as a result of reduced geranylgeranyl diphosphate levels. This study identified farnesyl disphosphate synthase as the mevalonate pathway enzyme inhibited by bisphosphonates. HPLC analysis of products from a liver cytosolic extract narrowed the potential targets for alendronate inhibition (IC(50) = 1700 nM) to isopentenyl diphosphate isomerase and farnesyl diphosphate synthase. Recombinant human farnesyl diphosphate synthase was inhibited by alendronate with an IC(50) of 460 nM (following 15 min preincubation). Alendronate did not inhibit isopentenyl diphosphate isomerase or GGPP synthase, partially purified from liver cytosol. Recombinant farnesyl diphosphate synthase was also inhibited by pamidronate (IC(50) = 500 nM) and risedronate (IC(50) = 3.9 nM), negligibly by etidronate (IC50 = 80 microM), and not at all by clodronate. In osteoclasts, alendronate inhibited the incorporation of [(3)H]mevalonolactone into proteins of 18-25 kDa and into nonsaponifiable lipids, including sterols. These findings (i) identify farnesyl diphosphate synthase as the selective target of alendronate in the mevalonate pathway, (ii) show that this enzyme is inhibited by other N-containing bisphosphonates, such as risendronate, but not by clodronate, supporting a different mechanism of action for different bisphosphonates, and (iii) document in purified osteoclasts alendronate inhibition of prenylation and sterol biosynthesis.


