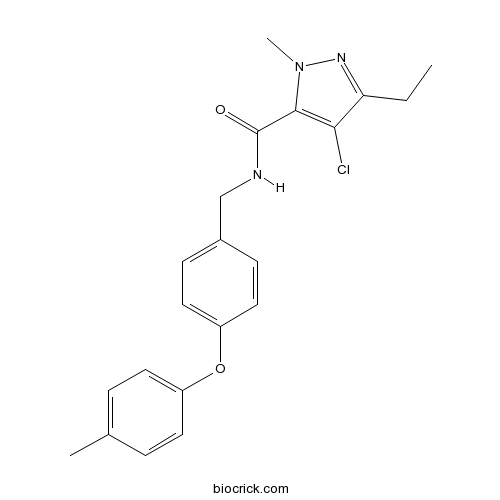
TolfenpyradCAS# 129558-76-5 |
- Entacapone sodium salt
Catalog No.:BCC4107
CAS No.:1047659-02-8
- Entacapone
Catalog No.:BCC2217
CAS No.:130929-57-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 129558-76-5 | SDF | Download SDF |
| PubChem ID | 10110536 | Appearance | Powder |
| Formula | C21H22ClN3O2 | M.Wt | 383.87 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 56 mg/mL (145.88 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-chloro-5-ethyl-2-methyl-N-[[4-(4-methylphenoxy)phenyl]methyl]pyrazole-3-carboxamide | ||
| SMILES | CCC1=NN(C(=C1Cl)C(=O)NCC2=CC=C(C=C2)OC3=CC=C(C=C3)C)C | ||
| Standard InChIKey | WPALTCMYPARVNV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H22ClN3O2/c1-4-18-19(22)20(25(3)24-18)21(26)23-13-15-7-11-17(12-8-15)27-16-9-5-14(2)6-10-16/h5-12H,4,13H2,1-3H3,(H,23,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tolfenpyrad is a pesticide that was first approved in 2002 in Japan under the trade name of Hachi-hachi. References: | |||||

Tolfenpyrad Dilution Calculator

Tolfenpyrad Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.605 mL | 13.0252 mL | 26.0505 mL | 52.101 mL | 65.1262 mL |
| 5 mM | 0.521 mL | 2.605 mL | 5.2101 mL | 10.4202 mL | 13.0252 mL |
| 10 mM | 0.2605 mL | 1.3025 mL | 2.605 mL | 5.2101 mL | 6.5126 mL |
| 50 mM | 0.0521 mL | 0.2605 mL | 0.521 mL | 1.042 mL | 1.3025 mL |
| 100 mM | 0.0261 mL | 0.1303 mL | 0.2605 mL | 0.521 mL | 0.6513 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tolfenpyrad is a pesticide that was first approved in 2002 in Japan under the trade name of Hachi-hachi.
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- 3,4-Diacetoxycinnamamide
Catalog No.:BCN6157
CAS No.:129488-34-2
- Fmoc-Asp-OtBu
Catalog No.:BCC3088
CAS No.:129460-09-9
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Boc-Cysteinol(pMeBzl)
Catalog No.:BCC3044
CAS No.:129397-85-9
- Fmoc-Phenylalaninol
Catalog No.:BCC2717
CAS No.:129397-83-7
- Euphorbia factor L9
Catalog No.:BCN3786
CAS No.:129393-28-8
- Physcion 8-O-rutinoside
Catalog No.:BCN7324
CAS No.:129393-21-1
- 11-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8430
CAS No.:129385-59-7
- O-Geranylconiferyl alcohol
Catalog No.:BCN4747
CAS No.:129350-09-0
- Alendronate sodium
Catalog No.:BCC3719
CAS No.:129318-43-0
- Licorisoflavan A
Catalog No.:BCN6662
CAS No.:129314-37-0
- Goniopypyrone
Catalog No.:BCN3957
CAS No.:129578-07-0
- Satraplatin
Catalog No.:BCC5356
CAS No.:129580-63-8
- Nevirapine
Catalog No.:BCC3820
CAS No.:129618-40-2
- GR 82334
Catalog No.:BCC5802
CAS No.:129623-01-4
- 3-Hydroxybisabola-1,10-dien-9-one
Catalog No.:BCN7325
CAS No.:129673-86-5
- 3,4-Dihydroxybisabola-1,10-diene
Catalog No.:BCN7326
CAS No.:129673-87-6
- ZD 7114 hydrochloride
Catalog No.:BCC6852
CAS No.:129689-28-7
- Dofequidar
Catalog No.:BCC4176
CAS No.:129716-58-1
- Aripiprazole
Catalog No.:BCC5034
CAS No.:129722-12-9
- 2',4'-Dihydroxy-3',6'-dimethoxychalcone
Catalog No.:BCN6158
CAS No.:129724-43-2
- 3-Hydroxylanost-9(11)-24-dien-26-oic acid
Catalog No.:BCN1586
CAS No.:129724-83-0
- Anemoside A3
Catalog No.:BCN2328
CAS No.:129724-84-1
Analysis of tolfenpyrad and its metabolites in plasma in a tolfenpyrad poisoning case.[Pubmed:22802573]
J Anal Toxicol. 2012 Sep;36(7):529-37.
Tolfenpyrad (TFP) is a pesticide that was first approved in 2002 in Japan under the trade name of Hachi-hachi. Analyses of TFP and its major metabolite, 4-[4-[(4-chloro-3-ethyl-1-methylpyrazol-5-yl)carbonylaminomethyl]phenoxy]benzoic acid (PTCA), in plasma obtained from a cadaver suspected to have died of TFP poisoning, were conducted by liquid chromatography-mass spectrometry. The existence of TFP and PTCA was confirmed by scan mode and quantitative analysis was performed by selected ion monitoring mode. Calibration curves showed good linearity over the range of 0.1-4 and 0.25-4 microg/mL, and concentrations were estimated to be 1.97 +/- 0.02 and 2.88 +/- 0.04 microg/mL for TFP and PTCA, respectively. The plasma extract was further examined to find other metabolites using quadrupole time-of-flight MS, and the results revealed three more metabolites, which were suggested to be hydroxy-TFP, dehydro-TFP and hydroxy-PTCA. Plausible metabolic pathways of TFP in humans are: (i) oxidation of the methyl group on the benzene ring, and (ii) hydroxylation followed by dehydration at the ethyl group on the pyrazole ring.
Acute fatal poisoning with Tolfenpyrad.[Pubmed:24237799]
J Forensic Leg Med. 2013 Nov;20(8):962-4.
The authors present a fatal case of poisoning with Tolfenpyrad (TFP), a pesticide first approved in Japan in 2002. A man in his fifties was found dead in the supine position at his son's home and the small towel with a smell of naphthalene was found nearby. Forensic autopsy was unremarkable, except for a very small amount of light pink fluid in the stomach, with naphthalene odour. The toxicological analyses revealed the presence of TFP and its major metabolite PTCA (4-[4-[(4-chloro-3-ethyl-1-methylpyrazol-5-yl)carbonylaminomethyl]phenoxy]benzoic acid), together with naphthalene and methyl naphthalenes in the post-mortem sample, with liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) respectively. The plasma concentrations of each substance were quantified as 1.97 mug/ml (TFP), 2.88 mug/ml (PTCA), 1.70 mug/ml (naphthalene), 0.67 mug/ml (1-methyl naphthalene) and 1.44 mug/ml (2-methyl naphthalene). According to these results together with autopsy findings, the cause of his death was determined to be acute Tolfenpyrad poisoning. This is the first case report of fatal poisoning attributable to an intake of TFP product.


