Entacapone sodium saltCOMT inhibitor CAS# 1047659-02-8 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- CCT137690
Catalog No.:BCC2188
CAS No.:1095382-05-0
- CYC116
Catalog No.:BCC2181
CAS No.:693228-63-6
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- TAK-901
Catalog No.:BCC2180
CAS No.:934541-31-8
- PF-03814735
Catalog No.:BCC2184
CAS No.:942487-16-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1047659-02-8 | SDF | Download SDF |
| PubChem ID | 78357787 | Appearance | Powder |
| Formula | C14H14N3NaO5 | M.Wt | 327.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
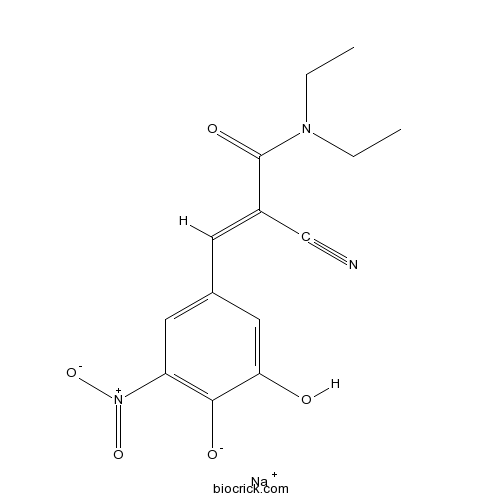
| Chemical Name | sodium;4-[(E)-2-cyano-3-(diethylamino)-3-oxoprop-1-enyl]-2-hydroxy-6-nitrophenolate | ||
| SMILES | CCN(CC)C(=O)C(=CC1=CC(=C(C(=C1)O)[O-])[N+](=O)[O-])C#N.[Na+] | ||
| Standard InChIKey | UMQUSXKIIWDDTK-OAZHBLANSA-M | ||
| Standard InChI | InChI=1S/C14H15N3O5.Na/c1-3-16(4-2)14(20)10(8-15)5-9-6-11(17(21)22)13(19)12(18)7-9;/h5-7,18-19H,3-4H2,1-2H3;/q;+1/p-1/b10-5+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Entacapone is a specific, potent, peripherally acting catechol-O-methyltransferase (COMT) inhibitor with IC50 of 151 nM for PD treatment.
IC50 Value: 151 nM
Target: COMT
in vitro: Entacapone inhibits catechol-O-methyltransferase(COMT) with similar IC50 in different tissues including live, duodenum, kidney and lung, but entacapone is more active than tolcapone in those tissues. Entacapone (< 100 μM) is a potent inhibitor of α-syn and β-amyloid (Aβ) oligomerization and fibrillogenesis, and also protects against extracellular toxicity induced by the aggregation of both proteins in PC12 cells.
in vivo: Levodopa/carbidopa/entacapone has been shown to improve the pharmacokinetic profile of levodopa and provide superior symptomatic control compared with conventional levodopa/dopa decarboxylase inhibitor therapy. We report four case histories describing clinical experience of using levodopa/carbidopa/entacapone 200/50/200 mg, one of the latest doses of this formulation, in a range of patients with Parkinson's disease. These cases illustrate that levodopa/carbidopa/entacapone 200/50/200 mg provides improvements in symptomatic control.
Clinical trial: The combination product carbidopa/levodopa/entacapone (CLE) was approved in 2003 for the treatment of PD patients. References: | |||||

Entacapone sodium salt Dilution Calculator

Entacapone sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0556 mL | 15.2779 mL | 30.5558 mL | 61.1116 mL | 76.3895 mL |
| 5 mM | 0.6111 mL | 3.0556 mL | 6.1112 mL | 12.2223 mL | 15.2779 mL |
| 10 mM | 0.3056 mL | 1.5278 mL | 3.0556 mL | 6.1112 mL | 7.639 mL |
| 50 mM | 0.0611 mL | 0.3056 mL | 0.6111 mL | 1.2222 mL | 1.5278 mL |
| 100 mM | 0.0306 mL | 0.1528 mL | 0.3056 mL | 0.6111 mL | 0.7639 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Entacapone, a nitrocatechol compound, is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT), a Mg2+-dependent enzyme involved in the metabolism of catecholamines and catechol compounds, with the half maximal inhibition concentration IC50 and inhibition constant Ki values of 20.1 nM and 10.7 nM respectively [1 & 2].
The nitro group at the position ortho to the hydroxyl group in the chemical structure of entacapone has been identified as a critical component for its potency and ability to inhibit COMT [2].
Entacapone is an FDA-approved COMT inhibitor for use as an adjunct to levodopa therapy in patients’ with Parkinson’s disease, where it increases both the peripheral and central availability of levodopa [2].
Reference
References:
[1] Forsberg M, Lehtonen M, Heikkinen M, Savolainen J, Järvinen T, Männistö PT. Pharmacokinetics and pharmacodynamics of entacapone and tolcapone after acute and repeated administration: a comparative study in the rat. J Pharmacol Exp Ther. 2003 Feb;304(2):498-506.
[2] Najib J. Entacapone: a catechol-O-methyltransferase inhibitor for the adjunctive treatment of Parkinson's disease. Clin Ther. 2001 Jun;23(6):802-32; discussion 771.
- Afuresertib
Catalog No.:BCC5502
CAS No.:1047644-62-1
- GSK2141795 hydrochloride
Catalog No.:BCC5295
CAS No.:1047635-80-2
- GSK2141795
Catalog No.:BCC5294
CAS No.:1047634-65-0
- Strontium chloride
Catalog No.:BCC7973
CAS No.:10476-85-4
- Ganoderic acid S
Catalog No.:BCN5861
CAS No.:104759-35-5
- 4E-Deacetylchromolaenide 4'-O-acetate
Catalog No.:BCN7263
CAS No.:104736-09-6
- Mayteine
Catalog No.:BCN3098
CAS No.:104736-05-2
- 6-O-α-Maltosyl-β-cyclodextrin
Catalog No.:BCC8075
CAS No.:104723-60-6
- 8-Phenyloctanol
Catalog No.:BCC8791
CAS No.:10472-97-6
- Boc-D-Glu(OtBu)-OH
Catalog No.:BCC3395
CAS No.:104719-63-3
- Ganoderal A
Catalog No.:BCN2451
CAS No.:104700-98-3
- Ganoderol A
Catalog No.:BCN5860
CAS No.:104700-97-2
- 3alpha-Akebonoic acid
Catalog No.:BCN5862
CAS No.:104777-61-9
- Plantamajoside
Catalog No.:BCN6279
CAS No.:104777-68-6
- Sappanone B
Catalog No.:BCN7942
CAS No.:104778-15-6
- 4-O-Methylsappanol
Catalog No.:BCN5863
CAS No.:104778-16-7
- MRS 2768 tetrasodium salt
Catalog No.:BCC7800
CAS No.:1047980-83-5
- Masitinib mesylate
Catalog No.:BCC1729
CAS No.:1048007-93-7
- COR 170
Catalog No.:BCC6282
CAS No.:1048039-15-1
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
- Germanicol acetate
Catalog No.:BCN7264
CAS No.:10483-91-7
- UPF 1069
Catalog No.:BCC2213
CAS No.:1048371-03-4
- NF 157
Catalog No.:BCC7367
CAS No.:104869-26-3
- NF 023
Catalog No.:BCC6985
CAS No.:104869-31-0
Gateways to clinical trials.[Pubmed:17003851]
Methods Find Exp Clin Pharmacol. 2006 Sep;28(7):451-95.
Gateways to Clinical Trials are a guide to the most recent clinical trials in current literature and congresses. The data in the following tables have been retrieved from the Clinical Trials Knowledge Area of Prous Science Integrity, the drug discovery and development portal, http://integrity.prous.com This issue focuses on the following selection of drugs: A-007, A6, adalimumab, adenosine triphosphate, alefacept, alemtuzumab, AllerVax Ragweed, amphora, anakinra, angiotensin-(1-7), anidulafungin, apomine, aripiprazole, atomoxetine hydrochloride, avanafil; BAL-8557, becatecarin, bevacizumab, biphasic insulin aspart, BMS-188797, bortezomib, bosentan, botulinum toxin type B, brivudine; Calcipotriol/betamethasone dipropionate, caspofungin acetate, catumaxomab, certolizumab pegol, cetuximab, CG-0070, ciclesonide, cinacalcet hydrochloride, clindamycin phosphate/benzoyl peroxide, cryptophycin 52, Cypher; Dabigatran etexilate, darapladib, darbepoetin alfa, decitabine, deferasirox, desloratadine, dexanabinol, dextromethorphan/quinidine sulfate, DMF, drotrecogin alfa (activated), duloxetine hydrochloride; E-7010, edaravone, efalizumab, emtricitabine, entecavir, eplerenone, erlotinib hydrochloride, escitalopram oxalate, estradiol valerate/dienogest, eszopiclone, exenatide, ezetimibe; Fondaparinux sodium, fulvestrant; Gefitinib, gestodene, GYKI-16084; Hyaluronic acid, hydralazine hydrochloride/isosorbide dinitrate; Imatinib mesylate, indiplon, insulin glargine; Juzen-taiho-to; Lamivudine/zidovudine/abacavir sulfate, L-arginine hydrochloride, lasofoxifene tartrate, L-BLP-25, lenalidomide, levocetirizine, levodopa/carbidopa/entacapone, lexatumumab, lidocaine/prilocaine, lubiprostone, lumiracoxib; MAb-14.18, mitoquidone; Natalizumab, neridronic acid, neuradiab; Olpadronic acid sodium salt, omalizumab; p53-DC vaccine, parathyroid hormone (human recombinant), peginterferon alfa-2a, peginterferon alfa-2b, pemetrexed disodium, perifosine, pimecrolimus, prasterone, prasugrel, PRO-2000, Pseudostat; R24, rasburicase, RHAMM R3 peptide, rilonacept, rosuvastatin calcium, rotavirus vaccine, rufinamide; Sabarubicin hydrochloride, SHL-749, sirolimus-eluting stent, SLx-2101, sodium butyrate, sorafenib, SU-6668; TachoSil, tadalafil, taxus, tegaserod maleate, telbivudine, tenofovir disoproxil fumarate, teriparatide, tetramethylpyrazine, teverelix, tiotropium bromide, tipifarnib, tirapazamine, tolvaptan, TransvaxTM hepatitis C vaccine, treprostinil sodium; Valganciclovir hydrochloride, valsartan/amlodipine, vandetanib, vardenafil hydrochloride hydrate, vatalanib succinate, veglin, voriconazole; Yttrium 90 (90Y) ibritumomab tiuxetan; Zileuton, zotarolimus, zotarolimus-eluting stent.
Gateways to clinical trials.[Pubmed:17200730]
Methods Find Exp Clin Pharmacol. 2006 Nov;28(9):657-78.
5-Methyltetrahydrofolate, (R)-flurbiprofen; Ad5CMV-p53, adalimumab, alefacept, alemtuzumab, Alequel, alicaforsen sodium, almotriptan, anakinra, aprepitant, aripiprazole, armodafinil; Bevacizumab, bortezomib, bosentan; Canfosfamide hydrochloride, ciclesonide, clofarabine, Cypher; Darbepoetin alfa, diclofenac potassium, drotrecogin alfa (activated), duloxetine hydrochloride; Eel calcitonin, eletriptan, eplerenone, everolimus, ezetimibe; Frovatriptan; Gefitinib, gamma-hydroxybutyrate sodium; HKI-272, HYB-165; Ibutamoren mesylate, imatinib mesylate, interleukin-21, ixabepilone; KRN-951; L-Arginine hydrochloride, levodopa/carbidopa/entacapone; Micafungin sodium, motexafin gadolinium, mycophenolic acid sodium salt; Nesiritide; Peginterferon alfa-2a, pitavastatin calcium, pralatrexate, pregabalin, pVAX/L523S-Ad.L523S; Rasagiline mesylate, recombinant human nerve growth factor, regadenoson, rF-PSA, rimonabant, rizatriptan, rofecoxib, rosuvastatin calcium, rV-B7.1, rV-PSA; Sipuleucel-T, sirolimus-eluting stent, solifenacin succinate, sorafenib, sunitinib malate; Talactoferrin alfa, Taxus, tegaserod maleate, teriparatide, tipifarnib; Valdecoxib, vandetanib, vatalanib succinate; WT1-peptide vaccine; Xaliproden hydrochloride. (c) 2006 Prous Science. All rights reserved.
Entacapone promotes cAMP-dependent colonic Cl(-) secretion in rats.[Pubmed:21501335]
Neurogastroenterol Motil. 2011 Jul;23(7):657-e277.
BACKGROUND: Entacapone is a promising drug used widely for the treatment of Parkinson's disease (PD) as a catechol-O-methyl transferase (COMT) inhibitor. However, entacapone has gastrointestinal side effects. The aim of this study was to investigate the effects of entacapone on the epithelial ion transport in rat distal colon, and explore the underlying mechanism. METHODS: The study was performed on freshly isolated colonic mucosa-only, submucosa-only and mucosa-submucosa preparations in rat. The short circuit current (I(SC) ) was measured to determine electrogenic ion transport, and a scanning ion-selective electrode technique (SIET) was used to directly measure Cl(-) flux across the epithelium. The content of intracellular cAMP was measured with radioimmunoassay (RIA). KEY RESULTS: Entacapone increased mucosal I(SC) in the rat distal colon. I(SC) was inhibited significantly by apical addition of diphenylamine-2,2'-dicarboxylic acid (DPC), a blocker of the Cl(-) channel, basolateral application of bumetanide, an inhibitor of Na(+) -K(+) -2Cl(-) co-transporter (NKCC), removal of Cl(-) from the bathing solution, and pretreatment with MDL 12330A, an inhibitor of adenylate cyclase. Inhibiting endogenous prostaglandin (PG) synthesis with indomethacin, and eliminating submucosal enteric neural activity with tetrodotoxin (TTX)-inhibited entacapone-evoked I(SC) increases. Similar results were also obtained when Cl(-) flux was measured with SIET. Entacapone significantly increased intracellular cAMP content, which was greatly inhibited by either indomethacin or TTX in the tissues containing submucosal plexus, and by only indomethacin in the mucosa-only preparations. CONCLUSIONS & INFERENCES: Entacapone stimulates cAMP-dependent Cl(-) secretion in the rat colon, and this process is regulated by endogenous PG and the submucosal enteric nervous system.
Gateways to clinical trials.[Pubmed:18040531]
Methods Find Exp Clin Pharmacol. 2007 Oct;29(8):547-83.
(-)-Epigallocatechin gallate, [188Re]-P2045, 12B75, 89-12; Abacavir sulfate/lamivudine, Abatacept, Abiraterone acetate, ABT-869, Adalimumab, Ad-rh Endostatin, AI-700, Alemtuzumab, Alvimopan hydrate, Amrubicin hydrochloride, AP-12009, Apomab 7.3, Arformoterol tartrate, Aripiprazole, AS-1404, Azacitidine, AZD-0530; Bevacizumab, BHT-3009, Biapenem, Bortezomib, Bosentan, Bremelanotide; CA9-SCAN, Calcitonin gene-related peptide, Canertinib dihydrochloride, Cannabidiol, Carboxyamidotriazole, Caspofungin acetate, Celgosivir, Certolizumab pegol, Cinacalcet hydrochloride, Clevudine, CP-751871, Curcumin, Cx-401, Cypher; Darunavir, Decitabine, Deforolimus, Dexamet, Dipyridamole/prednisolone, Drospirenone, Drospirenone/estradiol, DTPw-HepB-Hib, Duloxetine hydrochloride; Efalizumab, Emtricitabine, Erlotinib hydrochloride, Escitalopram oxalate, Eszopiclone; Ferumoxtran-10, Ferumoxytol, Fondaparinux sodium, Fosaprepitant dimeglumine; gamma-Hydroxybutyrate sodium, Gefitinib, Genistein, Ghrelin (human), Gimatecan, GM-CSF PMED, Golimumab, gp100 PMED; Imatinib mesylate, Immunoglobulin intravenous (human), IV Gamma-globulin; LA-419, Laropiprant, L-BLP-25, Levodopa/carbidopa/entacapone, Lidocaine/prilocaine, Lopinavir/ritonavir, Lumiracoxib, LY-2076962; Mepolizumab, Methylnaltrexone bromide, Mitiglinide calcium hydrate, Mycophenolic acid sodium salt, Myristyl nicotinate; Natalizumab, Nesiritide, Niacin/lovastatin; Oblimersen sodium, Ofatumumab, Olmesartan medoxomil, Olmesartan medoxomil/hydrochlorothiazide, Ozarelix; Palonosetron hydrochloride, Parathyroid hormone (human recombinant), Pazopanib hydrochloride, Pegaptanib octasodium, Pegfilgrastim, Peginterferon alfa- 2a, Peginterferon alfa-2b, Pegvisomant, Pemetrexed disodium, Pexelizumab, Picoplatin, Pimecrolimus, Posaconazole, Pregabalin, PRO-1762, Progesterone caproate, Prulifloxacin; Ramelteon, Ranelic acid distrontium salt, Reparixin, Rosuvastatin calcium; Rotigotine; Satraplatin, Sertraline, Sipuleucel-T, SLIT-cisplatin, SNDX-275, Solifenacin succinate, Sunitinib malate; Tadalafil, Talnetant, Tanespimycin, Taxus, Tegaserod maleate, Telmisartan/hydrochlorothiazide, Tenofovir disoproxil fumarate/emtricitabine, Teriparatide, tgAAC-94, Tiotropium bromide, Tocilizumab, Tolvaptan, Trimethoprim; Vardenafil hydrochloride hydrate, Vatalanib succinate, Vinflunine, Voriconazole, VX-680; XL-880; Yttrium 90 (90Y) ibritumomab tiuxetan.
Entacapone improves absorption of a coadministered salt in patients with Parkinson's disease.[Pubmed:18546331]
Mov Disord. 2008 Jul 30;23(10):1458-61.
Entacapone (EN) improves the efficacy of levodopa/dopadecarboxylase inhibitor (LD/DDI) formulations by inhibition of the enzyme catechol-O-methyltransferase (COMT). COMT inhibition also promotes the synthesis of basic LD metabolites, whereas DDI support the composition of acidic LD derivatives. LD metabolism correlates to the one of (13)C-sodium-octanoate, which is employed in breath tests to measure gastric emptying velocity. Objectives were to investigate the impact of COMT inhibition on the recovery rate of (13)C-sodium-octanoate in parkinsonian patients, who received first 100 mg LD/Carbidopa (CD) and the next day 100 mg LD/CD/EN combined with (13)C-sodium-octanoate in each case. The recovery rate of (13)C-sodium-octanoate was significant higher during the LD/CD/EN-compared with the LD/CD condition. COMT inhibition combined with LD/DDI improves absorption of a co-administered salt probably due to a COMT inhibition induced basic environment in gastrointestinal membranes. This improves dissolution and absorption of acids and salts. Thus it may enhance absorption of LD itself.


