COR 170Selective CB2 receptor inverse agonist CAS# 1048039-15-1 |
- Mozavaptan
Catalog No.:BCC5095
CAS No.:137975-06-5
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
- Desmopressin
Catalog No.:BCC1525
CAS No.:16679-58-6
- Desmopressin Acetate
Catalog No.:BCC1526
CAS No.:62288-83-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1048039-15-1 | SDF | Download SDF |
| PubChem ID | 25033736 | Appearance | Powder |
| Formula | C31H36N2O2 | M.Wt | 468.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 20 mM in DMSO | ||
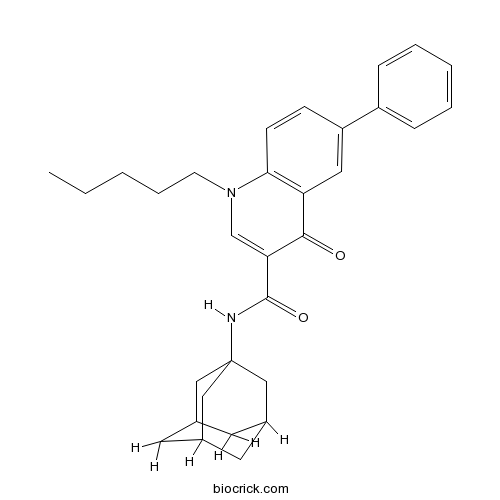
| Chemical Name | N-(1-adamantyl)-4-oxo-1-pentyl-6-phenylquinoline-3-carboxamide | ||
| SMILES | CCCCCN1C=C(C(=O)C2=C1C=CC(=C2)C3=CC=CC=C3)C(=O)NC45CC6CC(C4)CC(C6)C5 | ||
| Standard InChIKey | MSJISJDTJJYBFT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H36N2O2/c1-2-3-7-12-33-20-27(30(35)32-31-17-21-13-22(18-31)15-23(14-21)19-31)29(34)26-16-25(10-11-28(26)33)24-8-5-4-6-9-24/h4-6,8-11,16,20-23H,2-3,7,12-15,17-19H2,1H3,(H,32,35) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inverse agonist of CB2 receptors (Ki values are 3.8 and >10,000 nM for CB2 and CB1, respectively). |

COR 170 Dilution Calculator

COR 170 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1339 mL | 10.6694 mL | 21.3388 mL | 42.6776 mL | 53.347 mL |
| 5 mM | 0.4268 mL | 2.1339 mL | 4.2678 mL | 8.5355 mL | 10.6694 mL |
| 10 mM | 0.2134 mL | 1.0669 mL | 2.1339 mL | 4.2678 mL | 5.3347 mL |
| 50 mM | 0.0427 mL | 0.2134 mL | 0.4268 mL | 0.8536 mL | 1.0669 mL |
| 100 mM | 0.0213 mL | 0.1067 mL | 0.2134 mL | 0.4268 mL | 0.5335 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Masitinib mesylate
Catalog No.:BCC1729
CAS No.:1048007-93-7
- MRS 2768 tetrasodium salt
Catalog No.:BCC7800
CAS No.:1047980-83-5
- 4-O-Methylsappanol
Catalog No.:BCN5863
CAS No.:104778-16-7
- Sappanone B
Catalog No.:BCN7942
CAS No.:104778-15-6
- Plantamajoside
Catalog No.:BCN6279
CAS No.:104777-68-6
- 3alpha-Akebonoic acid
Catalog No.:BCN5862
CAS No.:104777-61-9
- Entacapone sodium salt
Catalog No.:BCC4107
CAS No.:1047659-02-8
- Afuresertib
Catalog No.:BCC5502
CAS No.:1047644-62-1
- GSK2141795 hydrochloride
Catalog No.:BCC5295
CAS No.:1047635-80-2
- GSK2141795
Catalog No.:BCC5294
CAS No.:1047634-65-0
- Strontium chloride
Catalog No.:BCC7973
CAS No.:10476-85-4
- Ganoderic acid S
Catalog No.:BCN5861
CAS No.:104759-35-5
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
- Germanicol acetate
Catalog No.:BCN7264
CAS No.:10483-91-7
- UPF 1069
Catalog No.:BCC2213
CAS No.:1048371-03-4
- NF 157
Catalog No.:BCC7367
CAS No.:104869-26-3
- NF 023
Catalog No.:BCC6985
CAS No.:104869-31-0
- (+)-Isopulegol
Catalog No.:BCN4975
CAS No.:104870-56-6
- dl-Aloesol
Catalog No.:BCN7265
CAS No.:104871-04-7
- Borapetoside B
Catalog No.:BCN6593
CAS No.:104901-05-5
- Tranilast Sodium
Catalog No.:BCC4091
CAS No.:104931-56-8
- H-D-Val-OtBu.HCl
Catalog No.:BCC3146
CAS No.:104944-18-5
- Ethyl β-D-ribo-hex-3-ulopyranoside
Catalog No.:BCC8977
CAS No.:104953-08-4
- 8-Hydroxydigitoxigenin
Catalog No.:BCN5864
CAS No.:1049674-06-7
A novel CB2 agonist, COR167, potently protects rat brain cortical slices against OGD and reperfusion injury.[Pubmed:23036353]
Pharmacol Res. 2012 Dec;66(6):555-63.
Cannabinoid CB2 receptor activation has been shown to have many pharmacological but not psychotropic effects. The aim of this study was to investigate the potential protection of brain tissues afforded by the novel substituted 4-quinolone-3-carboxylic acid derivative COR167, a selective CB2 agonist, toward ischemia and reperfusion-induced injury, as well as the mechanism of this potential effect. Rat brain cortical slices subjected to oxygen and glucose deprivation (OGD) followed by re-oxygenation were used. Cell damage was quantified by measuring at the end of the reperfusion phase the release into the artificial cerebrospinal fluid (ACSF) of lactate dehydrogenase (LDH), glutamate, IL-6 and TNF-alpha and by evaluating in tissue the lipid-peroxides (thiobarbituric acid-reactive substances, TBARS), the free, reduced glutathione content (GSH) and the water gain (TWG), taken as an index of cell swelling. COR167 (10nM or 100 nM), added to ACSF during the entire reperfusion phase, markedly reduced LDH and glutamate release, as well as TWG. Lower (0.1-1 nM) or higher concentrations (1,000 nM) were ineffective, suggesting thereby an hormetic behavior. COR167 at 10nM concentration markedly reverted in tissues TBARS increase and GSH decrease, while reducing IL-6 and TNF-alpha release into ACSF. COR167 effects on glutamate and LDH release were abrogated by the selective CB2 inverse-agonists COR170 (1 nM) and AM630 (1muM) but not by the CB1 antagonist AM251 (1 muM). COR170 as well as AM630 per se were able to revert TWG. The CB2 receptor agonist COR167 potently protected rat brain cortical slices against OGD and reperfusion injury, partly through CB2 receptors activation.
Investigations on the 4-quinolone-3-carboxylic acid motif. 2. Synthesis and structure-activity relationship of potent and selective cannabinoid-2 receptor agonists endowed with analgesic activity in vivo.[Pubmed:18680276]
J Med Chem. 2008 Aug 28;51(16):5075-84.
Quinolone-3-carboxamides 11 bearing at position 5, 6, 7, or 8 diverse substituents such as halides, alkyl, aryl, alkoxy, and aryloxy groups differing in their steric/electronic properties, were prepared. The new compounds were tested in vitro for CB1 and CB2 receptor affinity in comparison with the reference compounds rimonabant and SR144528. The tested compounds exhibited CB2 affinity in the range from 55.9 to 0.8 nM and CB1 affinity in the range from >10,000 to 5.3 nM, with selectivity indeces [Ki(CB1)/Ki(CB2)] varying from >2666.6 to 1.23. On the basis of the structure-selectivity relationship developed, the presence of a substituent at C6/C8 or C7 well accounts for the high or low CB2 selectivity, respectively. Compound 11c, characterized by high CB2 affinity and selectivity, showed analgesic activity in the formalin test of acute peripheral and inflammatory pain in mice as a result of selective CB2 agonistic activity.


