AfuresertibCAS# 1047644-62-1 |
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- Canertinib dihydrochloride
Catalog No.:BCC1449
CAS No.:289499-45-2
- CP-724714
Catalog No.:BCC1188
CAS No.:537705-08-1
- AC480 (BMS-599626)
Catalog No.:BCC1252
CAS No.:714971-09-2
- BMS-599626 Hydrochloride
Catalog No.:BCC1426
CAS No.:873837-23-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1047644-62-1 | SDF | Download SDF |
| PubChem ID | 46843057 | Appearance | Powder |
| Formula | C18H17Cl2FN4OS | M.Wt | 427.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GSK2110183C | ||
| Solubility | DMSO : ≥ 40 mg/mL (93.61 mM) *"≥" means soluble, but saturation unknown. | ||
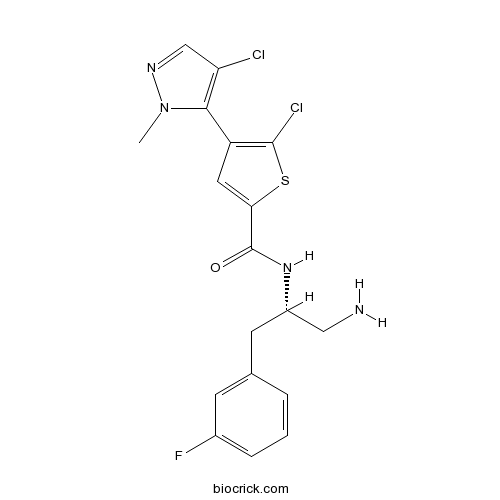
| Chemical Name | N-[(2S)-1-amino-3-(3-fluorophenyl)propan-2-yl]-5-chloro-4-(4-chloro-2-methylpyrazol-3-yl)thiophene-2-carboxamide | ||
| SMILES | CN1C(=C(C=N1)Cl)C2=C(SC(=C2)C(=O)NC(CC3=CC(=CC=C3)F)CN)Cl | ||
| Standard InChIKey | AFJRDFWMXUECEW-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C18H17Cl2FN4OS/c1-25-16(14(19)9-23-25)13-7-15(27-17(13)20)18(26)24-12(8-22)6-10-3-2-4-11(21)5-10/h2-5,7,9,12H,6,8,22H2,1H3,(H,24,26)/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Afuresertib Dilution Calculator

Afuresertib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3402 mL | 11.7008 mL | 23.4017 mL | 46.8033 mL | 58.5042 mL |
| 5 mM | 0.468 mL | 2.3402 mL | 4.6803 mL | 9.3607 mL | 11.7008 mL |
| 10 mM | 0.234 mL | 1.1701 mL | 2.3402 mL | 4.6803 mL | 5.8504 mL |
| 50 mM | 0.0468 mL | 0.234 mL | 0.468 mL | 0.9361 mL | 1.1701 mL |
| 100 mM | 0.0234 mL | 0.117 mL | 0.234 mL | 0.468 mL | 0.585 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Afuresertib is a potent and ATP-competitive specific Akt inhibitor.
In Vitro:Afuresertib exhibits favorable tumor-suppressive effects on malignant pleural mesothelioma (MPM) cells. Afuresertib significantly increases caspase-3 and caspase-7 activities and apoptotic cell number among ACC-MESO-4 and MSTO-211H cells. Afuresertib strongly arrests the cell cycle in the G1 phase. Western blotting analysis shows that Afuresertib increases the expression of p21WAF1/CIP1 and decreases the phosphorylation of Akt substrates, including GSK-3β and FOXO family proteins. Afuresertib-induced p21 expression promotes G1 phase arrest by inducing FOXO activity. Afuresertib significantly enhances cisplatin-induced cytotoxicity. Afuresertib modulates the expression E2F1 and MYC, which are associated with fibroblast core serum response[1].
References:
[1]. Yamaji M, et al. Novel ATP-competitive Akt inhibitor Afuresertib suppresses the proliferation of malignant pleural mesothelioma cells. Cancer Med. 2017 Nov;6(11):2646-2659.
- GSK2141795 hydrochloride
Catalog No.:BCC5295
CAS No.:1047635-80-2
- GSK2141795
Catalog No.:BCC5294
CAS No.:1047634-65-0
- Strontium chloride
Catalog No.:BCC7973
CAS No.:10476-85-4
- Ganoderic acid S
Catalog No.:BCN5861
CAS No.:104759-35-5
- 4E-Deacetylchromolaenide 4'-O-acetate
Catalog No.:BCN7263
CAS No.:104736-09-6
- Mayteine
Catalog No.:BCN3098
CAS No.:104736-05-2
- 6-O-α-Maltosyl-β-cyclodextrin
Catalog No.:BCC8075
CAS No.:104723-60-6
- 8-Phenyloctanol
Catalog No.:BCC8791
CAS No.:10472-97-6
- Boc-D-Glu(OtBu)-OH
Catalog No.:BCC3395
CAS No.:104719-63-3
- Ganoderal A
Catalog No.:BCN2451
CAS No.:104700-98-3
- Ganoderol A
Catalog No.:BCN5860
CAS No.:104700-97-2
- Ganoderol B
Catalog No.:BCN5859
CAS No.:104700-96-1
- Entacapone sodium salt
Catalog No.:BCC4107
CAS No.:1047659-02-8
- 3alpha-Akebonoic acid
Catalog No.:BCN5862
CAS No.:104777-61-9
- Plantamajoside
Catalog No.:BCN6279
CAS No.:104777-68-6
- Sappanone B
Catalog No.:BCN7942
CAS No.:104778-15-6
- 4-O-Methylsappanol
Catalog No.:BCN5863
CAS No.:104778-16-7
- MRS 2768 tetrasodium salt
Catalog No.:BCC7800
CAS No.:1047980-83-5
- Masitinib mesylate
Catalog No.:BCC1729
CAS No.:1048007-93-7
- COR 170
Catalog No.:BCC6282
CAS No.:1048039-15-1
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
- Germanicol acetate
Catalog No.:BCN7264
CAS No.:10483-91-7
- UPF 1069
Catalog No.:BCC2213
CAS No.:1048371-03-4
- NF 157
Catalog No.:BCC7367
CAS No.:104869-26-3
A phase IIa study of afuresertib, an oral pan-AKT inhibitor, in patients with Langerhans cell histiocytosis.[Pubmed:27804235]
Pediatr Blood Cancer. 2017 May;64(5).
BACKGROUND: Langerhans cell histiocytosis (LCH) is a clonal neoplasm characterized by widely varied clinical presentations, including multisystem involvement and systemic inflammatory symptoms. The AKT pathway is relevant to survival and proliferation of dendritic cells, and is also often upregulated in hematopoietic malignancies. A clinical response in an adult patient with LCH participating in the first-in-human trial of Afuresertib prompted this prospective trial. PROCEDURE: The population in the current study included treatment-naive (n = 7) and recurrent/refractory patients with LCH (n = 10), who received oral Afuresertib (125 mg). The majority of patients were treated for > 24 weeks, with four patients receiving treatment for > 48 weeks. RESULTS: Pharmacokinetic analysis showed similar exposures in previously reported patients with other hematologic malignancies. Primary drug-related toxicities included Grade 1/2 nausea, diarrhea, dyspepsia, and vomiting. Grade 3 toxicities included fatigue, diarrhea, and pain (one of each). Another severe adverse event involved soft tissue necrosis. The overall response rate in evaluable subjects was 33% in treatment-naive patients and 28% in patients with recurrent/refractory disease, which did not meet the predefined Bayesian criteria for efficacy. CONCLUSION: Afuresertib has clinical activity in some patients with newly diagnosed and advanced LCH.
The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma.[Pubmed:25075128]
Blood. 2014 Oct 2;124(14):2190-5.
The PI3K/AKT pathway is constitutively active in hematologic malignancies, providing proliferative and antiapoptotic signals and possibly contributing to drug resistance. We conducted an open-label phase 1 study to evaluate the maximum tolerated dose (MTD), safety, pharmacokinetics, and clinical activity of Afuresertib-an oral AKT inhibitor-in patients with advanced hematologic malignancies. Seventy-three patients were treated at doses ranging from 25 to 150 mg per day. The MTD was established at 125 mg per day because of 2 dose-limiting toxicities in the 150-mg cohort (liver function test abnormalities). The most frequent adverse events were nausea (35.6%), diarrhea (32.9%), and dyspepsia (24.7%). Maximum plasma concentrations and area under the plasma concentration-time curves from time 0 to 24 hours were generally dose proportional at > 75-mg doses; the median time to peak plasma concentrations was 1.5 to 2.5 hours post dose, with a half-life of approximately 1.7 days. Three multiple myeloma patients attained partial responses; an additional 3 attained minimal responses. Clinical activity was also observed in non-Hodgkin lymphoma, Langerhan's cell histiocytosis, and Hodgkin disease. Single-agent Afuresertib showed a favorable safety profile and demonstrated clinical activity against hematologic malignancies, including multiple myeloma.
Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma.[Pubmed:25417902]
Cancer Chemother Pharmacol. 2015 Jan;75(1):183-9.
PURPOSE: To identify the maximum tolerated dose (MTD) and recommended Phase II dose of MEK/AKT inhibitor combination of trametinib and Afuresertib. PATIENTS AND METHODS: Eligibility criteria were advanced solid tumors, 18 years or older, Eastern Cooperative Oncology Group performance status 0 or 1, and adequate organ function. Exclusion criteria included Type 1 diabetes, active GI disease, leptomeningeal disease, or current evidence/risk of retinal venous occlusion/central serous retinopathy. Clinical safety parameters and response were evaluated and analyzed. RESULTS: Twenty patients were enrolled. Dose-limiting toxicities (Grade 2 esophagitis; Grade 3 aspartate aminotransferase increased, mucosal inflammation and hypokalemia) were reported at starting dose (1.5 mg trametinib/50 mg Afuresertib once daily continuously), exceeding the MTD. Subsequent de-escalation cohorts (1.5 mg/25 mg or 1.0 mg/50 mg trametinib/Afuresertib) were defined as MTDs for continuous dosing. Intermittent dosing schedule [1.5 mg trametinib (continuous)/50 mg Afuresertib (Days 1-10 every 28 days)] was evaluated and considered tolerable. No patients were enrolled in Phase II. The most common adverse events reported (>/=10 % of all patients) included: diarrhea (60 %), dermatitis acneiform (55 %), maculo-papular rash (45 %), fatigue (30 %), dry skin (25 %), nausea (25 %), dyspnea (20 %), and vomiting (20 %). One partial response (BRAF wild-type melanoma) was reported; four patients had stable disease as best response. CONCLUSION: Continuous daily dosing of trametinib/Afuresertib combination was poorly tolerated. Evaluation of intermittent dose schedule showed greater tolerability. Given the interest in combination treatment regimens of MAPK and PI3K/AKT pathway inhibitors, further study of intermittent dose schedule or combination of trametinib with more selective inhibitors may be warranted.


