GSK2141795CAS# 1047634-65-0 |
- PP242
Catalog No.:BCC3682
CAS No.:1092351-67-1
- A66
Catalog No.:BCC3715
CAS No.:1166227-08-2
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- PIK-294
Catalog No.:BCC4995
CAS No.:900185-02-6
- OSI-027
Catalog No.:BCC4603
CAS No.:936890-98-1
- KU-0063794
Catalog No.:BCC2484
CAS No.:938440-64-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1047634-65-0 | SDF | Download SDF |
| PubChem ID | 51042438 | Appearance | Powder |
| Formula | C18H16Cl2F2N4O2 | M.Wt | 429.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Uprosertib | ||
| Solubility | DMSO : ≥ 150 mg/mL (349.45 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
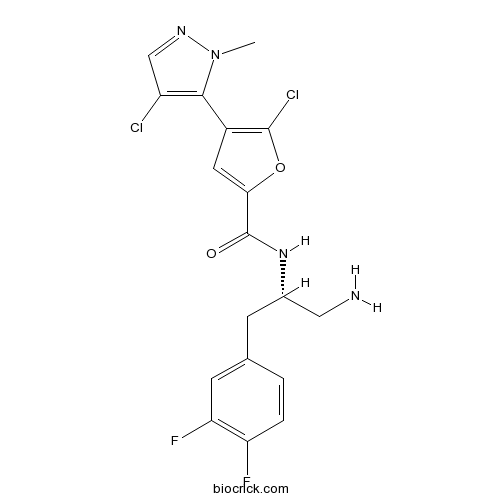
| Chemical Name | N-[(2S)-1-amino-3-(3,4-difluorophenyl)propan-2-yl]-5-chloro-4-(4-chloro-2-methylpyrazol-3-yl)furan-2-carboxamide | ||
| SMILES | CN1C(=C(C=N1)Cl)C2=C(OC(=C2)C(=O)NC(CC3=CC(=C(C=C3)F)F)CN)Cl | ||
| Standard InChIKey | AXTAPYRUEKNRBA-JTQLQIEISA-N | ||
| Standard InChI | InChI=1S/C18H16Cl2F2N4O2/c1-26-16(12(19)8-24-26)11-6-15(28-17(11)20)18(27)25-10(7-23)4-9-2-3-13(21)14(22)5-9/h2-3,5-6,8,10H,4,7,23H2,1H3,(H,25,27)/t10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

GSK2141795 Dilution Calculator

GSK2141795 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3296 mL | 11.6482 mL | 23.2964 mL | 46.5929 mL | 58.2411 mL |
| 5 mM | 0.4659 mL | 2.3296 mL | 4.6593 mL | 9.3186 mL | 11.6482 mL |
| 10 mM | 0.233 mL | 1.1648 mL | 2.3296 mL | 4.6593 mL | 5.8241 mL |
| 50 mM | 0.0466 mL | 0.233 mL | 0.4659 mL | 0.9319 mL | 1.1648 mL |
| 100 mM | 0.0233 mL | 0.1165 mL | 0.233 mL | 0.4659 mL | 0.5824 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK2141795 is a potent and selective pan-Akt inhibitor with IC50 values of 180/328/38 nM for Akt1/Akt2/Akt3, respectively.
In Vitro:GSK2141795 inhibits Akt1/2/3 with the Kd values of 16/49/5 nM, respectively. GSK2141795 potently inhibits only the PKC family members PRKACA and PRKACB as well as the cGMP-dependent protein kinase PRKG1 aqpart from the Akts. Protein targets that bind GSK2141795 in the lysate show a dose-dependent reduction in binding to the kinobeads, while proteins unaffected by the drug show no reduction in binding[1].
References:
[1]. Pachl F, et al. Characterization of a chemical affinity probe targeting Akt kinases. J Proteome Res. 2013 Aug 2;12(8):3792-800.
[2]. Jacobsen K, et al. Convergent Akt activation drives acquired EGFR inhibitor resistance in lung cancer. Nat Commun. 2017 Sep 4;8(1):410.
- Strontium chloride
Catalog No.:BCC7973
CAS No.:10476-85-4
- Ganoderic acid S
Catalog No.:BCN5861
CAS No.:104759-35-5
- 4E-Deacetylchromolaenide 4'-O-acetate
Catalog No.:BCN7263
CAS No.:104736-09-6
- Mayteine
Catalog No.:BCN3098
CAS No.:104736-05-2
- 6-O-α-Maltosyl-β-cyclodextrin
Catalog No.:BCC8075
CAS No.:104723-60-6
- 8-Phenyloctanol
Catalog No.:BCC8791
CAS No.:10472-97-6
- Boc-D-Glu(OtBu)-OH
Catalog No.:BCC3395
CAS No.:104719-63-3
- Ganoderal A
Catalog No.:BCN2451
CAS No.:104700-98-3
- Ganoderol A
Catalog No.:BCN5860
CAS No.:104700-97-2
- Ganoderol B
Catalog No.:BCN5859
CAS No.:104700-96-1
- Ganoderic acid K
Catalog No.:BCN3039
CAS No.:104700-95-0
- Lupiwighteone
Catalog No.:BCN4045
CAS No.:104691-86-3
- GSK2141795 hydrochloride
Catalog No.:BCC5295
CAS No.:1047635-80-2
- Afuresertib
Catalog No.:BCC5502
CAS No.:1047644-62-1
- Entacapone sodium salt
Catalog No.:BCC4107
CAS No.:1047659-02-8
- 3alpha-Akebonoic acid
Catalog No.:BCN5862
CAS No.:104777-61-9
- Plantamajoside
Catalog No.:BCN6279
CAS No.:104777-68-6
- Sappanone B
Catalog No.:BCN7942
CAS No.:104778-15-6
- 4-O-Methylsappanol
Catalog No.:BCN5863
CAS No.:104778-16-7
- MRS 2768 tetrasodium salt
Catalog No.:BCC7800
CAS No.:1047980-83-5
- Masitinib mesylate
Catalog No.:BCC1729
CAS No.:1048007-93-7
- COR 170
Catalog No.:BCC6282
CAS No.:1048039-15-1
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
- Germanicol acetate
Catalog No.:BCN7264
CAS No.:10483-91-7
Dose-Finding Quantitative 18F-FDG PET Imaging Study with the Oral Pan-AKT Inhibitor GSK2141795 in Patients with Gynecologic Malignancies.[Pubmed:26429956]
J Nucl Med. 2015 Dec;56(12):1828-35.
UNLABELLED: AKT (a serine/threonine-specific protein kinase) regulates many cellular processes contributing to cytotoxic drug resistance. This study's primary objective examined the relationship between GSK2141795, an oral, pan-AKT inhibitor, and (18)F-FDG PET markers of glucose metabolism in tumor tissue to determine whether (18)F-FDG PET could be used to guide personalized dosing of GSK2141795. Biomarker analysis of biopsies was also undertaken. METHODS: Twelve patients were enrolled in 3 cohorts; all underwent dynamic (18)F-FDG PET scans and serial pharmacokinetic sampling at baseline, week 2, and week 4 with tumor biopsies before treatment and at week 4. Response was evaluated by RECIST v1.1 and Gynecologic Cancer Intergroup criteria. Biopsy samples were analyzed for mutations and protein expression. RESULTS: GSK2141795 did not significantly influence blood glucose levels. No dose-response relationship was observed between GSK2141795 pharmacokinetics and (18)F-FDG PET pharmacodynamic measures; however, an exposure-response relationship was seen between maximum drug concentrations and maximal decrease in (18)F-FDG uptake in the best-responding tumor. This relationship also held for pharmacokinetic parameters of exposure and 1,5-anhydroglucitol (a systemic measure of glucose metabolism). Phospho-AKT upregulation at week 4 in biopsies confirmed AKT inhibition by GSK2141795. Single-agent activity was observed with a clinical benefit rate of 27% (3/11) and 30% (3/10) CA125 response in the study's platinum-resistant ovarian patients. AKT pathway activation by PIK3CA/PIK3R1 mutation did not correlate with clinical activity, whereas RAS/RAF pathway mutations did segregate with resistance to AKT inhibition. CONCLUSION: GSK2141795 demonstrated an exposure-response relationship with decreased (18)F-FDG uptake and is active and tolerable. This study's design integrating (18)F-FDG PET, pharmacokinetics, and biomarker analyses demonstrates the potential for clinical development for personalized treatment.


