Sappanone BCAS# 104778-15-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104778-15-6 | SDF | Download SDF |
| PubChem ID | 13888976 | Appearance | Powder |
| Formula | C16H14O6 | M.Wt | 302.28 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
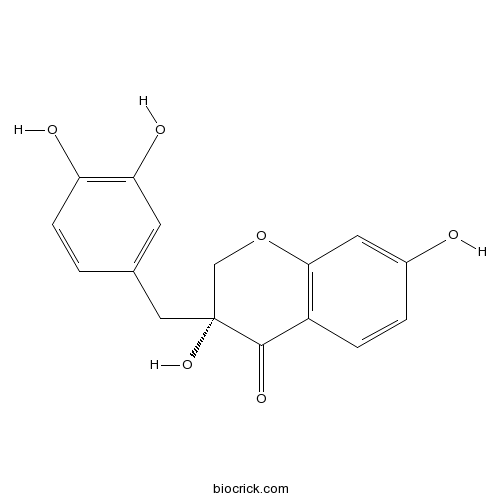
| Chemical Name | (3R)-3-[(3,4-dihydroxyphenyl)methyl]-3,7-dihydroxy-2H-chromen-4-one | ||
| SMILES | C1C(C(=O)C2=C(O1)C=C(C=C2)O)(CC3=CC(=C(C=C3)O)O)O | ||
| Standard InChIKey | BTLMXNHNFFXBHW-MRXNPFEDSA-N | ||
| Standard InChI | InChI=1S/C16H14O6/c17-10-2-3-11-14(6-10)22-8-16(21,15(11)20)7-9-1-4-12(18)13(19)5-9/h1-6,17-19,21H,7-8H2/t16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Sappanone B exhibits moderate to weak activity against methicillin-susceptible S. aureus (MSSA) and other standard strains by MICs/MBCs ranged from 32/64 to >1024/>1024 ug/ml. 2. Sappanone B has vasorelaxation effects, it exhibits an acute relaxation either in endothelium-intact or endothelium-denuded rings in a concentration-dependent manner. |
| Targets | NOS | NO |

Sappanone B Dilution Calculator

Sappanone B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3082 mL | 16.541 mL | 33.0819 mL | 66.1638 mL | 82.7048 mL |
| 5 mM | 0.6616 mL | 3.3082 mL | 6.6164 mL | 13.2328 mL | 16.541 mL |
| 10 mM | 0.3308 mL | 1.6541 mL | 3.3082 mL | 6.6164 mL | 8.2705 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3233 mL | 1.6541 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Plantamajoside
Catalog No.:BCN6279
CAS No.:104777-68-6
- 3alpha-Akebonoic acid
Catalog No.:BCN5862
CAS No.:104777-61-9
- Entacapone sodium salt
Catalog No.:BCC4107
CAS No.:1047659-02-8
- Afuresertib
Catalog No.:BCC5502
CAS No.:1047644-62-1
- GSK2141795 hydrochloride
Catalog No.:BCC5295
CAS No.:1047635-80-2
- GSK2141795
Catalog No.:BCC5294
CAS No.:1047634-65-0
- Strontium chloride
Catalog No.:BCC7973
CAS No.:10476-85-4
- Ganoderic acid S
Catalog No.:BCN5861
CAS No.:104759-35-5
- 4E-Deacetylchromolaenide 4'-O-acetate
Catalog No.:BCN7263
CAS No.:104736-09-6
- Mayteine
Catalog No.:BCN3098
CAS No.:104736-05-2
- 6-O-α-Maltosyl-β-cyclodextrin
Catalog No.:BCC8075
CAS No.:104723-60-6
- 8-Phenyloctanol
Catalog No.:BCC8791
CAS No.:10472-97-6
- 4-O-Methylsappanol
Catalog No.:BCN5863
CAS No.:104778-16-7
- MRS 2768 tetrasodium salt
Catalog No.:BCC7800
CAS No.:1047980-83-5
- Masitinib mesylate
Catalog No.:BCC1729
CAS No.:1048007-93-7
- COR 170
Catalog No.:BCC6282
CAS No.:1048039-15-1
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
- Germanicol acetate
Catalog No.:BCN7264
CAS No.:10483-91-7
- UPF 1069
Catalog No.:BCC2213
CAS No.:1048371-03-4
- NF 157
Catalog No.:BCC7367
CAS No.:104869-26-3
- NF 023
Catalog No.:BCC6985
CAS No.:104869-31-0
- (+)-Isopulegol
Catalog No.:BCN4975
CAS No.:104870-56-6
- dl-Aloesol
Catalog No.:BCN7265
CAS No.:104871-04-7
- Borapetoside B
Catalog No.:BCN6593
CAS No.:104901-05-5
[Vasorelaxation effects of homoisoflavonoids from Caesalpinia sappan in rat thoracic aortic rings].[Pubmed:19624017]
Zhongguo Zhong Yao Za Zhi. 2009 Mar;34(6):731-4.
OBJECTIVE: To identify and elucidate the vasorelaxant activity of homoisoflavonoids, the main chemical components from Lignum Sappan (the stems of Caesalpinia sappan), in isolated rat thoracic aortic rings pre-contracted with phenylephrine (PE, 1 micromol x L(-1)) and KCl (60 mmol x L(-1)). METHOD: The tension of rat thoracic aorta rings was used to evaluated the vasorelaxant activities of four homoisoflavonoids, brazlin (1), (E)-3-(3,4-dihydroxybenzylidene)-7-hydroxychroman-4-one (2), Sappanone B (3), 3-deoxySappanone B (4). RESULT: Cumulative addition of homoisoflavonoids (2, 3 and 4) (50-1000 micromol x L(-1)) exhibited an acute relaxation either in endothelium-intact or endothelium-denuded rings in a concentration-dependent manner. However, this relaxation was significantly inhibited in endothelium-denuded condition and in the presence of endothelial nitric oxide synthase (eNOS) inhibitor, N(W)-nitro-L-arginine methyl ester (L-NNA, 100 micromol x L(-1)), and a soluble guanylate cylcase (sGC) inhibitor, methylene blue (MB, 10 micromol x L(-1)) when addition of variation homoisoflavonoids brazlin (1) (50-1000 micromol x L(-1)). CONCLUSION: These results indicate that normo-homoisoflavonoids (2, 3 and 4) from Caesalpinia sappan mediates endothelium-independent vasodilator action in rat thoracic aortic rings, while the variation homoisoflavonoids brazlin elicits endothelium-dependent relaxation might via nitric oxide (NO)-cGMP pathway. This research could explain the pharmacological activities of homoisoflavonoids to a certain degree.
Synergy of aminoglycoside antibiotics by 3-Benzylchroman derivatives from the Chinese drug Caesalpinia sappan against clinical methicillin-resistant Staphylococcus aureus (MRSA).[Pubmed:24703330]
Phytomedicine. 2014 Jun 15;21(7):936-41.
The in vitro antimicrobial activities of three 3-Benzylchroman derivatives, i.e. Brazilin (1), Brazilein (2) and Sappanone B (3) from Caesalpinia sappan L. (Leguminosae) were assayed, which mainly dealt with synergistic evaluation of aminoglycoside and other type of antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) by the three compounds through the Chequerboard and Time-kill curve methods. The results showed that Compounds 1-3 alone exhibited moderate to weak activity against methicillin-susceptible S. aureus (MSSA) and other standard strains by MICs/MBCs ranged from 32/64 to >1024/>1024 mug/ml, with the order of activity as 1>2>3. Chequerboard method showed significant anti-MRSA synergy of 1/Aminoglycosides (Gentamicin, Amikacin, Etimicin and Streptomycin) combinations with (FICIs)50 at 0.375-0.5. The combined (MICs)50 values (mug/ml) reduced from 32-128/16-64 to 4-8/4-16, respectively. The percent of reduction by MICs ranged from 50% to 87.5%, with a maximum of 93.8% (1/16 of the alone MIC). Combinations of 2 and 3 with Aminoglycosides and the other antibiotics showed less potency of synergy. The dynamic Time-killing experiment further demonstrated that the combinations of 1/aminoglycoside were synergistically bactericidal against MRSA. The anti-MRSA synergy results of the bacteriostatic (Chequerboard method) and bactericidal (time-kill method) efficiencies of 1/Aminoglycoside combinations was in good consistency, which made the resistance reversed by CLSI guidelines. We concluded that the 3-Benzylchroman derivative Brazilin (1) showed in vitro synergy of bactericidal activities against MRSA when combined with Aminoglycosides, which might be beneficial for combinatory therapy of MRSA infection.


