(+)-IsopulegolCAS# 104870-56-6 |
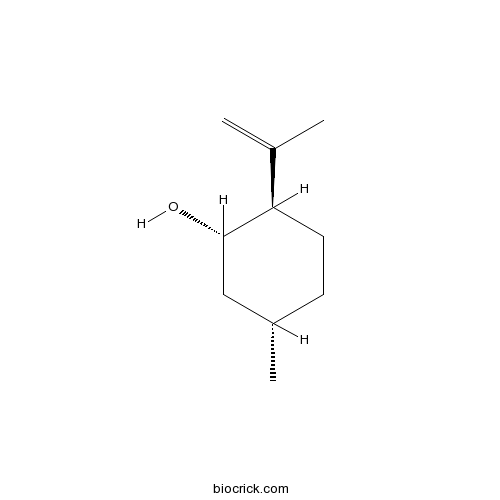
- Isopulegol
Catalog No.:BCN4974
CAS No.:7786-67-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104870-56-6 | SDF | Download SDF |
| PubChem ID | 1268090 | Appearance | Oil |
| Formula | C10H18O | M.Wt | 154.25 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,2R,5S)-5-methyl-2-prop-1-en-2-ylcyclohexan-1-ol | ||
| SMILES | CC1CCC(C(C1)O)C(=C)C | ||
| Standard InChIKey | ZYTMANIQRDEHIO-AEJSXWLSSA-N | ||
| Standard InChI | InChI=1S/C10H18O/c1-7(2)9-5-4-8(3)6-10(9)11/h8-11H,1,4-6H2,2-3H3/t8-,9+,10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Chemcatchem, 2017.Selectivity in the Cyclization of Citronellal Introduced by Squalene Hopene Cyclase Variants.[Reference: WebLink]The squalene hopene cyclase from Alicyclobacillus acidocaldarius (AacSHC) is a highly efficient enzyme catalyst for stereoselective Brønsted acid catalysis. |

(+)-Isopulegol Dilution Calculator

(+)-Isopulegol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.483 mL | 32.4149 mL | 64.8298 mL | 129.6596 mL | 162.0746 mL |
| 5 mM | 1.2966 mL | 6.483 mL | 12.966 mL | 25.9319 mL | 32.4149 mL |
| 10 mM | 0.6483 mL | 3.2415 mL | 6.483 mL | 12.966 mL | 16.2075 mL |
| 50 mM | 0.1297 mL | 0.6483 mL | 1.2966 mL | 2.5932 mL | 3.2415 mL |
| 100 mM | 0.0648 mL | 0.3241 mL | 0.6483 mL | 1.2966 mL | 1.6207 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NF 023
Catalog No.:BCC6985
CAS No.:104869-31-0
- NF 157
Catalog No.:BCC7367
CAS No.:104869-26-3
- UPF 1069
Catalog No.:BCC2213
CAS No.:1048371-03-4
- Germanicol acetate
Catalog No.:BCN7264
CAS No.:10483-91-7
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
- COR 170
Catalog No.:BCC6282
CAS No.:1048039-15-1
- Masitinib mesylate
Catalog No.:BCC1729
CAS No.:1048007-93-7
- MRS 2768 tetrasodium salt
Catalog No.:BCC7800
CAS No.:1047980-83-5
- 4-O-Methylsappanol
Catalog No.:BCN5863
CAS No.:104778-16-7
- Sappanone B
Catalog No.:BCN7942
CAS No.:104778-15-6
- Plantamajoside
Catalog No.:BCN6279
CAS No.:104777-68-6
- 3alpha-Akebonoic acid
Catalog No.:BCN5862
CAS No.:104777-61-9
- dl-Aloesol
Catalog No.:BCN7265
CAS No.:104871-04-7
- Borapetoside B
Catalog No.:BCN6593
CAS No.:104901-05-5
- Tranilast Sodium
Catalog No.:BCC4091
CAS No.:104931-56-8
- H-D-Val-OtBu.HCl
Catalog No.:BCC3146
CAS No.:104944-18-5
- Ethyl β-D-ribo-hex-3-ulopyranoside
Catalog No.:BCC8977
CAS No.:104953-08-4
- 8-Hydroxydigitoxigenin
Catalog No.:BCN5864
CAS No.:1049674-06-7
- Naspm trihydrochloride
Catalog No.:BCC7476
CAS No.:1049731-36-3
- A 331440 dihydrochloride
Catalog No.:BCC7963
CAS No.:1049740-32-0
- Cardiogenol C hydrochloride
Catalog No.:BCC7790
CAS No.:1049741-55-0
- PS 1145 dihydrochloride
Catalog No.:BCC7949
CAS No.:1049743-58-9
- 3-Acetoxy-4,7(11)-cadinadien-8-one
Catalog No.:BCN5865
CAS No.:104975-02-2
- Tacrolimus (FK506)
Catalog No.:BCC4952
CAS No.:104987-11-3
Chemoenzymatic Synthesis of the Intermediates in the Peppermint Monoterpenoid Biosynthetic Pathway.[Pubmed:29979593]
J Nat Prod. 2018 Jul 27;81(7):1546-1552.
A chemoenzymatic approach providing access to all four intermediates in the peppermint biosynthetic pathway between limonene and menthone/isomenthone, including noncommercially available intermediates (-)- trans-isopiperitenol (2), (-)-isopiperitenone (3), and (+)- cis-isopulegone (4), is described. Oxidation of (+)-Isopulegol (13) followed by enolate selenation and oxidative elimination steps provides (-)-isopiperitenone (3). A chemical reduction and separation route from (3) provides both native (-)- trans-isopiperitenol (2) and isomer (-)- cis-isopiperitenol (18), while enzymatic conjugate reduction of (-)-isopiperitenone (3) with IPR [(-)-isopiperitenone reductase)] provides (+)- cis-isopulegone (4). This undergoes facile base-mediated chemical epimerization to (+)-pulegone (5), which is subsequently shown to be a substrate for NtDBR ( Nicotiana tabacum double-bond reductase) to afford (-)-menthone (7) and (+)-isomenthone (8).
One-step, stereoselective synthesis of octahydrochromanes via the Prins reaction and their cannabinoid activities.[Pubmed:29880989]
Tetrahedron Lett. 2018 Feb 28;59(9):807-810.
Novel, functionalized octahydrochromene derivatives were synthesized in a single step via the Prins reaction. Enantiomerically pure (+)-Isopulegol was reacted with benzaldehyde to stereoselectively yield the corresponding octahydro-2H-chromen-4-ol derivative containing five stereocenters. A total of 10 compounds were synthesized by altering the enantiomer of isopulegol and the substituted benzaldehyde, and the resulting enantiopure octahydrochromenes were screened in vitro against the cannabinoid receptor isoforms CB1 and CB2. Compounds containing an olefin at the C4 position [(+)-3c, (-)-3c, (-)-7c, (-)-9c and (-)-11c] of the octahydrochromene scaffold were found to exhibit reasonable displacement of [(3)H] CP55,940 from the CB receptors, whereas the corresponding hydroxy analogs [(+)-3a, (+)-3b, (-)-3a, (-)-3b and (+)-5a] had very little or no effect.


