Masitinib mesylateTyrosine kinase inhibitor CAS# 1048007-93-7 |
- Tyrphostin AG 1296
Catalog No.:BCC1195
CAS No.:146535-11-7
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1048007-93-7 | SDF | Download SDF |
| PubChem ID | 25024769 | Appearance | Powder |
| Formula | C29H34N6O4S2 | M.Wt | 594.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AB-1010 mesylate | ||
| Solubility | DMSO : ≥ 30 mg/mL (50.44 mM) *"≥" means soluble, but saturation unknown. | ||
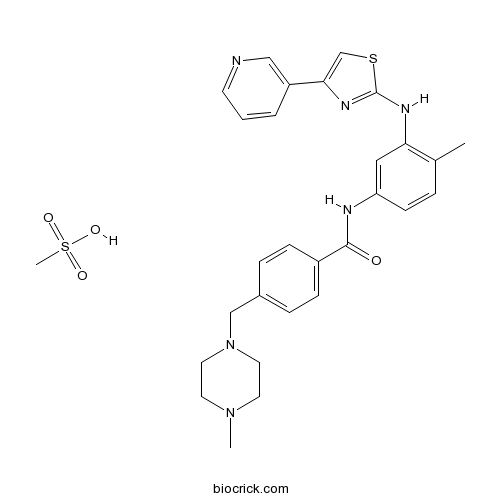
| Chemical Name | methanesulfonic acid;4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-yl-1,3-thiazol-2-yl)amino]phenyl]benzamide | ||
| SMILES | CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC(=CS4)C5=CN=CC=C5.CS(=O)(=O)O | ||
| Standard InChIKey | TXCWBWKVIZGWEQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H30N6OS.CH4O3S/c1-20-5-10-24(16-25(20)31-28-32-26(19-36-28)23-4-3-11-29-17-23)30-27(35)22-8-6-21(7-9-22)18-34-14-12-33(2)13-15-34;1-5(2,3)4/h3-11,16-17,19H,12-15,18H2,1-2H3,(H,30,35)(H,31,32);1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Masitinib mesylate is a novel inhibitor for Kit and PDGFRα/β with IC50 of 200 nM and 540 nM/800 nM, and has weak inhibition to ABL and c-Fms.In Vitro:Masitinib is a competitive inhibitor against ATP at concentrations ≤500 nM. Masitinib also potently inhibits recombinant PDGFR and the intracellular kinase Lyn, and to a lesser extent, fibroblast growth factor receptor 3. In contrast, masitinib demonstrates weak inhibition of Abl and c-Fms. Masitinib more strongly inhibits degranulation, cytokine production, and bone marrow mast cell migration than imatinib. In Ba/F3 cells expressing human wild-type Kit, masitinib inhibits SCF (stem cell factor)-induced cell proliferation with an IC50 of 150 nM, while the IC50 for inhibition of IL-3-stimulated proliferation is at approximately >10 µM. In Ba/F3 cells expressing PDGFRα, masitinib inhibits PDGF-BB-stimulated proliferation and PDGFRα tyrosine phosphorylation with IC50 of 300 nM. Masitinib also causes inhibition of SCF-stimulated tyrosine phosphorylation of human Kit in mastocytoma cell-lines and BMMC. Masitinib inhibits Kit gain-of-function mutants, including V559D mutant and Δ27 mouse mutant with IC50 of 3 and 5 nM in Ba/F3 cells. Masitinib inhibits the cell proliferation of mastocytoma cell lines including HMC-1α155 and FMA3 with IC50 of 10 and 30 nM, respectively[1]. Masitinib inhibits cell growth and PDGFR phosphorylation in two novel ISS cell lines, which suggest that Masitinib displays activity against both primary and metastatic ISS cell line and may aid in the clinical management of ISS[2].In Vivo:Masitinib inhibits tumour growth and increases the median survival time in Δ27-expressing Ba/F3 tumor models at 30 mg/kg, without cardiotoxicity or genotoxicity[1]. Masitinib (12.5 mg/kg/d, p.o.) increases overall TTP (time-to-tumor progression) compared with placebo in dogs[3]. The combination of masitinib/gemcitabine shows synergy in vitro on proliferation of gemcitabine-refractory cell lines Mia Paca2 and Panc1, and to a lesser extent on Mia Paca-2 pancreatic tumours in mice[4]. References: | |||||

Masitinib mesylate Dilution Calculator

Masitinib mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6814 mL | 8.4069 mL | 16.8138 mL | 33.6276 mL | 42.0345 mL |
| 5 mM | 0.3363 mL | 1.6814 mL | 3.3628 mL | 6.7255 mL | 8.4069 mL |
| 10 mM | 0.1681 mL | 0.8407 mL | 1.6814 mL | 3.3628 mL | 4.2034 mL |
| 50 mM | 0.0336 mL | 0.1681 mL | 0.3363 mL | 0.6726 mL | 0.8407 mL |
| 100 mM | 0.0168 mL | 0.0841 mL | 0.1681 mL | 0.3363 mL | 0.4203 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 200±40 nM ( recombinant human wild-type KIT); 540±60 (PDGFRα) [1] Masitinib, a tyrosine kinase inhibitor approved for treatment of canine mast cell tumours, is highly selective for the PDGFR signalling pathway and may offer a new therapeutic approach for this disease. in vitro: Masitinib inhibited the recombinant enzyme with a half inhibitory concentration (IC50) of 200±40 nM. Kinetic studies in which ATP and masitinib were covaried showed that at concentrations ≤500 nM masitinib is a competitive inhibitor against ATP, but at higher concentrations (>1 ?M), it has a mixed mechanism of inhibition against ATP. Under identical assay conditions and with the same enzyme, imatinib had an IC50 of 470±120 nM and was a strictly competitive inhibitor against ATP. Masitinib only weakly inhibited the proliferation of Ba/F3 cells expressing the D816V mutant of KIT, which is associated with adult mastocytosis and myeloproliferative disorder-acute myeloid leukaemia (exon 17), with an IC50 of 5.0±2.0 ?M [1]. The 50% inhibitory concentration (IC(50) ) at 72 h for three HSA cell lines (DEN, Fitz and SB) was found to be 8.56, 9.41 and 10.65 ?M, respectively. Further investigation demonstrated that masitinib mesylate induced apoptosis in all HSA cell lines, including activation of caspase-3/7. Measurement of VEGF levels in cell supernatant found a statistically significant increased VEGF in close proximity to the IC(50) of each cell line followed by a decline back towards baseline [2]. in vivo: Masitinib appeared to have a positive effect on MS-related impairment for PPMS and rfSPMS patients, as evidenced by an improvement in MSFC scores relative to baseline, compared with a worsening MSFC score in patients receiving placebo; +103% ± 189 versus -60% ± 190 at month-12, respectively [3]. A reduction in CAD Extent and Severity Index (CADESI-02) score of ≥ 50% at week 12 was observed in 61% of masitinib-treated dogs versus 35% of control dogs (P < 0.001), according to the modified intent-to-treat population [4]. Toxicity: Clinically relevant proteinuria was noted in 2/20 (10%) cats (both treated daily), and neutropenia was noted in 3/20 (15%) (seen in both treatment groups). An increase in serum creatinine concentration and adverse gastrointestinal effects were noted in some cats [5]. Clinical trial: N/A
- MRS 2768 tetrasodium salt
Catalog No.:BCC7800
CAS No.:1047980-83-5
- 4-O-Methylsappanol
Catalog No.:BCN5863
CAS No.:104778-16-7
- Sappanone B
Catalog No.:BCN7942
CAS No.:104778-15-6
- Plantamajoside
Catalog No.:BCN6279
CAS No.:104777-68-6
- 3alpha-Akebonoic acid
Catalog No.:BCN5862
CAS No.:104777-61-9
- Entacapone sodium salt
Catalog No.:BCC4107
CAS No.:1047659-02-8
- Afuresertib
Catalog No.:BCC5502
CAS No.:1047644-62-1
- GSK2141795 hydrochloride
Catalog No.:BCC5295
CAS No.:1047635-80-2
- GSK2141795
Catalog No.:BCC5294
CAS No.:1047634-65-0
- Strontium chloride
Catalog No.:BCC7973
CAS No.:10476-85-4
- Ganoderic acid S
Catalog No.:BCN5861
CAS No.:104759-35-5
- 4E-Deacetylchromolaenide 4'-O-acetate
Catalog No.:BCN7263
CAS No.:104736-09-6
- COR 170
Catalog No.:BCC6282
CAS No.:1048039-15-1
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
- Germanicol acetate
Catalog No.:BCN7264
CAS No.:10483-91-7
- UPF 1069
Catalog No.:BCC2213
CAS No.:1048371-03-4
- NF 157
Catalog No.:BCC7367
CAS No.:104869-26-3
- NF 023
Catalog No.:BCC6985
CAS No.:104869-31-0
- (+)-Isopulegol
Catalog No.:BCN4975
CAS No.:104870-56-6
- dl-Aloesol
Catalog No.:BCN7265
CAS No.:104871-04-7
- Borapetoside B
Catalog No.:BCN6593
CAS No.:104901-05-5
- Tranilast Sodium
Catalog No.:BCC4091
CAS No.:104931-56-8
- H-D-Val-OtBu.HCl
Catalog No.:BCC3146
CAS No.:104944-18-5
- Ethyl β-D-ribo-hex-3-ulopyranoside
Catalog No.:BCC8977
CAS No.:104953-08-4
ALS Clinical Trials Review: 20 Years of Failure. Are We Any Closer to Registering a New Treatment?[Pubmed:28382000]
Front Aging Neurosci. 2017 Mar 22;9:68.
Amyotrophic lateral sclerosis (ALS) is a devastating condition with an estimated mortality of 30,000 patients a year worldwide. The median reported survival time since onset ranges from 24 to 48 months. Riluzole is the only currently approved mildly efficacious treatment. Riluzole received marketing authorization in 1995 in the USA and in 1996 in Europe. In the years that followed, over 60 molecules have been investigated as a possible treatment for ALS. Despite significant research efforts, the overwhelming majority of human clinical trials (CTs) have failed to demonstrate clinical efficacy. In the past year, oral masitinib and intravenous edaravone have emerged as promising new therapeutics with claimed efficacy in CTs in ALS patients. Given their advanced phase of clinical development one may consider these drugs as the most likely near-term additions to the therapeutic arsenal available for patients with ALS. In terms of patient inclusion, CT with masitinib recruited a wider, more representative, less restrictive patient population in comparison to the only successful edaravone CT (edaravone eligibility criteria represents only 18% of masitinib study patients). The present manuscript reviews >50 CTs conducted in the last 20 years since riluzole was first approved. A special emphasis is put on the analysis of existing evidence in support of the clinical efficacy of edaravone and masitinib and the possible implications of an eventual marketing authorisation in the treatment of ALS.
Estrogen-induced transcription factor EGR1 regulates c-Kit transcription in the mouse uterus to maintain uterine receptivity for embryo implantation.[Pubmed:28965972]
Mol Cell Endocrinol. 2018 Jul 15;470:75-83.
Early growth response 1 (Egr1) is a key transcription factor that mediates the action of estrogen (E2) to establish uterine receptivity for embryo implantation. However, few direct target genes of EGR1 have been identified in the uterus. Here, we demonstrated that E2 induced EGR1-regulated transcription of c-Kit, which plays a crucial role in cell fate decisions. Spatiotemporal expression of c-Kit followed that of EGR1 in uteri of ovariectomized mice at various time points after E2 treatment. E2 activated ERK1/2 and p38 to induce EGR1, which then activated c-Kit expression in the uterus. EGR1 transfection produced rapid and transient induction of c-KIT in a time- and dose-dependent manner. Furthermore, luciferase assays to measure c-Kit promoter activity confirmed that a functional EGR1 binding site(s) (EBS) was located within -1 kb of the c-Kit promoter. Site-directed mutagenesis and chromatin immunoprecipitation-PCR for three putative EBS within -1 kb demonstrated that the EBS at -818/-805 was critical for EGR1-dependent c-Kit transcription. c-Kit expression was significantly increased in the uterus on day 4 and administration of Masitinib, a c-Kit inhibitor, effectively interfered with embryo implantation. Collectively, our results showed that estrogen induces transcription factor EGR1 to regulate c-Kit transcription for uterine receptivity for embryo implantation in the mouse uterus.
The JAK2/STAT5 signaling pathway as a potential therapeutic target in canine mastocytoma.[Pubmed:28397975]
Vet Comp Oncol. 2018 Mar;16(1):55-68.
BACKGROUND: Mastocytoma are frequently diagnosed cutaneous neoplasms in dogs. In non-resectable mastocytoma patients, novel targeted drugs are often applied. The transcription factor STAT5 has been implicated in the survival of human neoplastic mast cells (MC). Our study evaluated the JAK2/STAT5 pathway as a novel target in canine mastocytoma. MATERIALS AND METHODS: We employed inhibitors of JAK2 (R763, TG101348, AZD1480, ruxolitinib) and STAT5 (pimozide, piceatannol) and evaluated their effects on 2 mastocytoma cell lines, C2 and NI-1. RESULTS: Activated JAK2 and STAT5 were detected in both cell lines. The drugs applied were found to inhibit proliferation and survival in these cells with the following rank-order of potency: R763 > TG101348 > AZD1480 > pimozide > ruxolitinib > piceatannol. Moreover, synergistic anti-neoplastic effects were obtained by combining pimozide with KIT-targeting drugs (toceranib, masitinib, nilotinib, midostaurin) in NI-1 cells. CONCLUSION: The JAK2/STAT5 pathway is a novel potential target of therapy in canine mastocytoma.
Can binimetinib, encorafenib and masitinib be more efficacious than currently available mutation-based targeted therapies for melanoma treatment?[Pubmed:28277830]
Expert Opin Pharmacother. 2017 Apr;18(5):487-495.
INTRODUCTION: Historically, there were few effective and durable treatments for metastatic melanoma. Recently, mutation based targeted therapies have revolutionized treatment and outcomes for patients with metastatic melanoma. Specifically, inhibitors aimed at BRAF, NRAS, and C-KIT mutations are now commonly used in treatment for patients harboring the specific mutations. Areas covered: A brief review of current BRAF, NRAS, and C-KIT inhibitors provides background for a thorough review of newly developed agents namely binimetinib, a MEK inhibitor, encorafenib a BRAF inhibitor, and masitinib which inhibits C-KIT. Expert opinion: While the 3 novel agents reviewed here have potential for use in melanoma, optimal utilization will occur once a more personalized approach incorporating genomic, proteomic, and immunologic data guides therapeutic decisions.


