Imatinib Mesylate (STI571)Abl/c-kit/PDGFR inhibitor CAS# 220127-57-1 |
- DCC-2036 (Rebastinib)
Catalog No.:BCC4390
CAS No.:1020172-07-9
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- GNF 2
Catalog No.:BCC3891
CAS No.:778270-11-4
- GNF 5
Catalog No.:BCC3892
CAS No.:778277-15-9
- Ponatinib (AP24534)
Catalog No.:BCC2522
CAS No.:943319-70-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220127-57-1 | SDF | Download SDF |
| PubChem ID | 123596 | Appearance | Powder |
| Formula | C30H35N7O4S | M.Wt | 589.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Gleevec, CGP 57148B | ||
| Solubility | H2O : ≥ 50 mg/mL (84.79 mM) DMSO : ≥ 49 mg/mL (83.09 mM) *"≥" means soluble, but saturation unknown. | ||
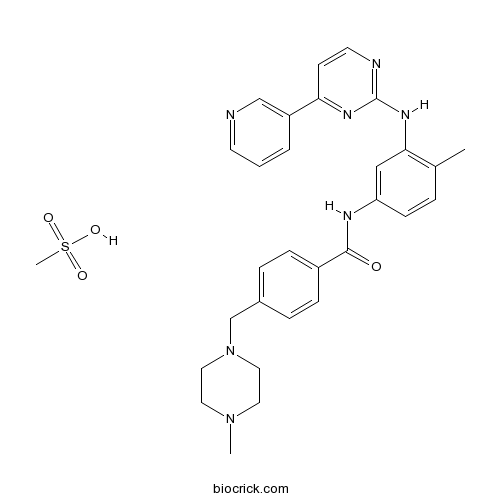
| Chemical Name | methanesulfonic acid;4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide | ||
| SMILES | CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5.CS(=O)(=O)O | ||
| Standard InChIKey | YLMAHDNUQAMNNX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H31N7O.CH4O3S/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36;1-5(2,3)4/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34);1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Imatinib Mesylate is a known inhibitor of the c-Kit, Bcr-Abl, and PDGFR tyrosine kinases, inhibits the SLF-dependent activation of c-Kitwt kinase with IC50 of ~100 nM, which is similar to the concentration requires for inhibition of Bcr-Abl and PDGFR.In Vitro:Imatinib (STI571) Mesylate inhibits c-Kit autophosphorylation, activation of MAPK, and activation of Akt without altering total protein levels of c-kit, MAPK, or Akt. The concentration that produces 50% inhibition for these effects is approximately 100 nM[1]. Imatinib (STI571) mesylate is very effective (in vitro IC50 of 25 nM) against the chronic myeloid leukemia-causing kinase Bcr-Abl. Imatinib also efficiently inhibits Kit (in vitro IC50, 410 nM) and PDGFR (in vitro IC50, 380 nM)[2]. Imatinib (STI571) mesylate is a multi-target inhibitor of v-Abl, c-Kit and inhibits Bcr/Abl, v-Abl, Tel/Abl, the native PDGFβ receptor, and c-Kit, but it does not inhibit Src family kinases, c-Fms, Flt3, the EGFR or multiple other tyrosine kinases. Imatinib inhibits tyrosine phosphorylation and cell growth of Ba/F3 cells expressing Bcr/Abl, Tel/Abl, Tel/PDGFβR, and Tel/Arg with an IC50 of approximately 0.5 μM in each case, but it has no effect on untransformed Ba/F3 cells growing in IL-3 or on Ba/F3 cells transformed by Tel/JAK2[3]. Imatinib mesylate selectively inhibits the activity of Bcr/Abl, c-Kit and PDGFR kinases. Imatinib mesylate reveals distinct and rapid antileukemic activity in chronic myelogenous leukemia (CML) and Philadelphia-positive (Ph+) acute lymphoblastic leukemia (ALL)[4].In Vivo:Animals treated with Imatinib Mesylate show a decrease of mean body weight throughout the whole study. Body weight loss is noticeable in mice from groups that receive chemotherapy and the vitamin D analog combined treatment. The body weight decrease of mice treat with both combined Imatinib mesylate and PRI-2191 is the highest (15%) on Day 22 of the experiment, but after that day, mice start to recover[4]. In a rat Ischemia/reperfusion injury (IRI) model, Imatinib mesylate attenuates lung injury by an antipermeability and antiinflammatory effect. The delivery and function of Imatinib mesylate in the lung is also confirmed in this model[5]. References: | |||||
| Cell experiment: [1] | |
| Cell lines | T cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | IC50: 3.9 μM for inhibiting DCs-stimulated T-cell proliferation 2.9 μM for inhibiting PHA-stimulated T-cell proliferation 4 days |
| Applications | Cells were stimulated with allogeneic mature DCs or PHA in the presence of imatinib mesylate. The drug inhibited T-cell proliferation as a function of concentration. The effects were significant at 0.5 μM imatinib mesylate for the cells stimulated by DCs and at 1.0 μM imatinib mesylate for the cells stimulated with PHA. The IC50 values for imatinib mesylate–inhibited T-cell proliferation stimulated by DCs and PHA were 3.9 μM and 2.9 μM, respectively. |
| Animal experiment: [2] | |
| Animal models | Female C57BL/6 mice |
| Dosage form | Intraperitoneal injection, 25 or 50mg/kg/day |
| Application | Administration of imatinib alone did not generate any changes in lung morphology. However, when imatinib was administered in bleomycin-treated mice, a reduction of fibrotic lesions in the subpleural areas of lung was observed at doses of 25 and 50 mg/kg/day. The quantitative histologic analysis demonstrated that the fibrotic score in mice treated with bleomycin and 50 mg/kg/day of imatinib was significantly lower than that treated with bleomycin alone. The collagen content of the lung was also significantly lower in mice treated with bleomycin and imatinib (50 mg/kg/day) as compared with those treated with bleomycin alone. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Dietz A B, Souan L, Knutson G J, et al. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood, 2004, 104(4): 1094-1099. [2] Aono Y, Nishioka Y, Inayama M, et al. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. American journal of respiratory and critical care medicine, 2005, 171(11): 1279-1285. | |

Imatinib Mesylate (STI571) Dilution Calculator

Imatinib Mesylate (STI571) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6957 mL | 8.4787 mL | 16.9575 mL | 33.915 mL | 42.3937 mL |
| 5 mM | 0.3391 mL | 1.6957 mL | 3.3915 mL | 6.783 mL | 8.4787 mL |
| 10 mM | 0.1696 mL | 0.8479 mL | 1.6957 mL | 3.3915 mL | 4.2394 mL |
| 50 mM | 0.0339 mL | 0.1696 mL | 0.3391 mL | 0.6783 mL | 0.8479 mL |
| 100 mM | 0.017 mL | 0.0848 mL | 0.1696 mL | 0.3391 mL | 0.4239 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
The anti-proliferative and pro-apoptotic activities of Imatinib Mesylate against CML cells can be enhanced by Wnt5a.
Abstract
Although inconsistent results have been obtained from studies examining functional role of tyrosine kinase receptors in the generation of spontaneous activity in various segments of the gastrointestinal and urogenital tracts through imatinib mesylate, effects of imatinib mesylate on the spontaneous activity in the young and ageing prostate gland have been reported in detail.
Abstract
New treatment regimens for MPNSTs are needed.
Abstract
Imatinib mesylate has been evaluated for its efficacy to decrease the volume burden of plexiforn neurofibromas in NF1 patients.
Abstract
Imatinib mesylate, a tyrosine kinase inhibitor with anticancer activity, causes cardiotoxicity in patients.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Imatinib mesylate is a tyrosine kinase inhibitor IC50 value of 100 nM, 100 nM, 600 nM for v-Abl, c-kit, PDGFR, respectively [1].
Tyrosine kinase is an enzyme which is a subclass of protein kinase and plays an important role in transferring a phosphate group from ATP to a protein in cells. It is shown that tyrosine kinase plays a pivotal role in the management of disorders in which activation of c-Abl, PDGFR, or c-Kit signaling. Recently, the role of tyrosine kinases in the modulation of growth factor signaling are received more and more attention and gradually become an especial important target [2].
Imatinib Mesylate is a specific tyrosine kinase (abl, c-kit, and PDGFR) inhibitor and is reported to sensitize cells to radio- or chemo-therapy. When tested with Y-79 and WERI-RB-1 Rb cell lines, imatinib mesylate treatment decreased the cell proliferation and invasion with the concentration of 10 μM [3]. In osteoblast cells, administration of imatinib mesylate decreased osteoclast development via stimulating differentiation, inhibiting proliferation and survival [4].
In dog model with mast cell tumor, administration of imatinib mesylate at a dose of 10 mg/kg daily for 1-9 weeks reduced tumor growth via inhibiting tyrosine kinase [5].
References:
[1].Buchdunger, E., et al., Selective inhibition of the platelet-derived growth factor signal transduction pathway by a protein-tyrosine kinase inhibitor of the 2-phenylaminopyrimidine class. Proc Natl Acad Sci U S A, 1995. 92(7): p. 2558-62.
[2].Zhou, Y., et al., The multi-targeted tyrosine kinase inhibitor vandetanib plays a bifunctional role in non-small cell lung cancer cells. Sci Rep, 2015. 5: p. 8629.
[3].de Moura, L.R., et al., The effect of imatinib mesylate on the proliferation, invasive ability, and radiosensitivity of retinoblastoma cell lines. Eye (Lond), 2013. 27(1): p. 92-9.
[4].O'Sullivan, S., et al., Imatinib mesylate does not increase bone volume in vivo. Calcif Tissue Int, 2011. 88(1): p. 16-22.
[5].Isotani, M., et al., Effect of tyrosine kinase inhibition by imatinib mesylate on mast cell tumors in dogs. J Vet Intern Med, 2008. 22(4): p. 985-8.
- Vincetoxicoside B
Catalog No.:BCN2864
CAS No.:22007-72-3
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- Ixabepilone
Catalog No.:BCC1666
CAS No.:219989-84-1
- Fmoc-β-Homo-Tyr(tBu)-OH
Catalog No.:BCC2621
CAS No.:219967-69-8
- Isoescin IB
Catalog No.:BCN2969
CAS No.:219944-46-4
- Isoescin IA
Catalog No.:BCN2968
CAS No.:219944-39-5
- 3-Methyl-1-(2-piperidinophenyl)butylamine N-acetylglutamate salt
Catalog No.:BCC8635
CAS No.:219921-94-5
- 5,8,9,14-Tetraacetoxy-3-benzoyloxy-10,15-dihydroxypepluane
Catalog No.:BCN7657
CAS No.:219916-77-5
- MPEP Hydrochloride
Catalog No.:BCC1777
CAS No.:219911-35-0
- 2,2',3'-Trihydroxy-4,6-dimethoxybenzophenone
Catalog No.:BCN1488
CAS No.:219861-73-1
- Escitalopram Oxalate
Catalog No.:BCC5040
CAS No.:219861-08-2
- Merresectine B
Catalog No.:BCN1918
CAS No.:219829-75-1
- Eichlerialactone
Catalog No.:BCN4941
CAS No.:2202-01-9
- O-Methyldauricine
Catalog No.:BCC8225
CAS No.:2202-17-7
- Triptohairic acid
Catalog No.:BCN8060
CAS No.:220209-71-2
- Cinnamamide
Catalog No.:BCN4942
CAS No.:22031-64-7
- 5-[2-[Tert-butyl(dimethyl)silyl]oxyethyl]-2,2-dimethyl-3a,6a-dihydrofuro[2,3-d][1,3]dioxol-6-one
Catalog No.:BCC8592
CAS No.:220328-03-0
- 3,11,12-Trihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1487
CAS No.:220328-04-1
- TRO 19622
Catalog No.:BCC5288
CAS No.:22033-87-0
- DH 97
Catalog No.:BCC6973
CAS No.:220339-00-4
- 5-Epicanadensene
Catalog No.:BCN7349
CAS No.:220384-17-8
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
- Polycephalin C
Catalog No.:BCN1852
CAS No.:220422-37-7
- Kaempferol 5-methyl ether
Catalog No.:BCN3426
CAS No.:22044-80-0
The novel pyrrolo-1,5-benzoxazepine, PBOX-21, potentiates the apoptotic efficacy of STI571 (imatinib mesylate) in human chronic myeloid leukaemia cells.[Pubmed:19014913]
Biochem Pharmacol. 2009 Feb 1;77(3):310-21.
The Bcr-Abl kinase inhibitor, STI571, is the first line treatment for chronic myeloid leukaemia (CML), but the recent emergence of STI571 resistance has led to the examination of combination therapies. In this report, we describe how a novel non-toxic G1-arresting compound, pyrrolo-1,5-benzoxazepine (PBOX)-21, potentiates the apoptotic ability of STI571 in Bcr-Abl-positive CML cells. Co-treatment of CML cells with PBOX-21 and STI571 induced more apoptosis than either drug alone in parental (K562S and LAMA84) and STI571-resistant cells lines (K562R). This potentiation of apoptosis was specific to Bcr-Abl-positive leukaemia cells with no effect observed on Bcr-Abl-negative HL-60 acute myeloid leukaemia cells. Apoptosis induced by PBOX-21/STI571 resulted in activation of caspase-8, cleavage of PARP and Bcl-2, upregulation of the pro-apoptotic protein Bim and a downregulation of Bcr-Abl. Repression of proteins involved in Bcr-Abl transformation, the anti-apoptotic proteins Mcl-1 and Bcl-(XL) was also observed. The combined lack of an early change in mitochondrial membrane potential, release of cytochrome c and cleavage of pro-caspase-9 suggests that this pathway is not involved in the initiation of apoptosis by PBOX-21/STI571. Apoptosis was significantly reduced following pre-treatment with either the general caspase inhibitor Boc-FMK or the chymotrypsin-like serine protease inhibitor TPCK, but was completely abrogated following pre-treatment with a combination of these inhibitors. This demonstrates the important role for each of these protease families in this apoptotic pathway. In conclusion, our data highlights the potential of PBOX-21 in combination with STI571 as an effective therapy against CML.
A phase I dose-escalation study of imatinib mesylate (Gleevec/STI571) plus capecitabine (Xeloda) in advanced solid tumors.[Pubmed:20530436]
Anticancer Res. 2010 Apr;30(4):1251-6.
UNLABELLED: The aim of this study was to determine the maximally tolerated dose, recommended phase II dose and toxicity profile of capecitabine plus imatinib mesylate combination. PATIENTS AND METHODS: Twenty-four patients with advanced solid tumors were treated with capecitabine twice daily on days 1-14 and imatinib mesylate once daily on a 21-day cycle. Dose-limiting toxicity was assessed during the first cycle. Treatment continued until disease progression or undesirable toxicity. RESULTS: Six patients were treated with capecitabine at 1000 mg/m(2) and imatinib mesylate 300 mg; unacceptable toxicity due to grade 2 intolerable hand-foot syndrome and/or grade > or = 2 diarrhea was observed. Doses were subsequently reduced to capecitabine at 750 mg/m(2) and imatinib mesylate at 300 mg; toxicities were better tolerated at the lower dose. Dose-limiting toxicities consisted of grade 3 diarrhea, anorexia and fatigue lasting > or = 4 days. Treatment-related adverse events greater than or equal to grade 3 included anemia, diarrhea, dysuria, hypophosphatemia and vertigo. Minor responses were observed in two patients: stable disease > or = 6 months was observed in two out of twenty-one evaluable patients. CONCLUSION: Full doses of capecitabine and imatinib mesylate were not tolerable. The maximum tolerated dose and the recommended phase II dose for this drug combination is capecitabine at 750 mg/m(2) twice daily for 1-14 days and imatinib at 300 mg once daily on a 21-day cycle.
Imatinib mesylate (STI571) enhances amrubicin-induced cytotoxic activity through inhibition of the phosphatidylinositol 3-kinase/Akt pathway in small cell lung cancer cells.[Pubmed:19956885]
Oncol Rep. 2010 Jan;23(1):217-22.
Small cell lung cancer (SCLC) is characterized by autocrine mechanisms. Stem cell factor (SCF) and its receptor c-kit can activate Akt and extracellular signal-regulated kinase (Erk) pathways. Imatinib Mesylate (STI571) can inhibit c-kit tyrosine kinase activity, but clinical trials have resulted in failure. We investigated the possibility of SCF/c-kit-targeted therapy against SCLC. Using c-kit-positive SCLC cells (H209 and H69 cells) and SCF as a model of the autocrine mechanisms, the effects of SCF, LY294002, PD98059 or STI571 on Akt and Erk were assessed by Western blot analysis. The cell growth inhibitions of cisplatin, etoposide irinotecan and amrubicin (AMR) with or without SCF, LY294002, PD98059 or STI571 were evaluated by MTT assay. Treatment with SCF activated Akt and Erk and the activations were inhibited by STI571 in H209 but not in H69 cells. LY294002 and PD98059 inhibited SCF-induced Akt and Erk activation in H209 cells, respectively. STI571 alone did not exert growth inhibition in the SCF-treated cells. In H209 cells, SCF decreased the cytotoxicity of AMR, but not of other drugs. In H69 cells, SCF did not affect sensitivity to any drugs. LY294002 but not PD98059 restored or enhanced AMR-sensitivity in SCF-treated H209 or untreated H69 cells, respectively. STI571 restored the AMR-sensitivity of SCF-treated H209 cells to the basal level. If the SCF/c-kit contributes to Akt activation in vivo, the combination of STI571 and AMR may be effective against SCLC. Additionally, using a combination of AKT inhibitors and AMR may be a promising treatment in the future.
Local delivery of imatinib mesylate (STI571)-incorporated nanoparticle ex vivo suppresses vein graft neointima formation.[Pubmed:18824771]
Circulation. 2008 Sep 30;118(14 Suppl):S65-70.
BACKGROUND: Clinical outcome of surgical revascularization using autologous vein graft is limited by vein graft failure attributable to neointima formation. Platelet-derived growth factor (PDGF) plays a central role in the pathogenesis of vein graft failure. Therefore, we hypothesized that nanoparticle (NP)-mediated drug delivery system of PDGF-receptor (PDGF-R) tyrosine kinase inhibitor (imatinib mesylate: STI571) could be an innovative therapeutic strategy. METHODS AND RESULTS: Uptake of STI571-NP normalized PDGF-induced cell proliferation and migration. Excised rabbit jugular vein was treated ex vivo with PBS, STI571 only, FITC-NP, or STI571-NP, then interposed back into the carotid artery position. NP was detected in many cells in the neointima and media at 7 and 28 days after grafting. Significant neointima was formed 28 days after grafting in the PBS group; this neointima formation was suppressed in the STI571-NP group. STI571-NP treatment inhibited cell proliferation and phosphorylation of the PDGF-R-beta but did not affect inflammation and endothelial regeneration. CONCLUSIONS: STI571-NP-induced suppression of vein graft neointima formation holds promise as a strategy for preventing vein graft failure.
Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis.[Pubmed:16014680]
Ann Rheum Dis. 2005 Aug;64(8):1126-31.
BACKGROUND: Mast cells have been implicated in the pathogenesis of arthritis, but elucidation of their precise role has been hampered by a lack of efficient and selective inhibitors of their function. OBJECTIVE: To elucidate the role of mast cells in the pathogenesis of rheumatoid arthritis (RA) and to assess whether apoptosis of cultured and synovial tissue mast cells can be induced by inhibiting mast cell growth factor receptor, c-kit tyrosine kinase. METHODS AND RESULTS: Double staining with tumour necrosis factor (TNF) alpha and tryptase antibodies showed the presence of TNFalpha positive mast cells in human rheumatoid synovial tissue. Selective activation of mast cells by anti-IgE resulted in production of TNFalpha in synovial tissue cultures. Inhibition of the c-kit tyrosine kinase with imatinib mesylate (1.0-10 micromol/l) induced profound apoptosis in cultured mast cells as judged by typical apoptotic morphology, increased number of apoptotic nucleosomes, and activation of caspases 8 and 9. Importantly, imatinib also induced apoptosis of mast cells in explant cultures of synovial tissue obtained from patients with RA as judged by a TUNEL assay. Inhibition of c-kit tyrosine kinase was accompanied by significant reduction of TNFalpha production in synovial tissue cultures. CONCLUSION: Mast cells may have a role in the pathogenesis of RA, and inhibition of c-kit may be a new means of inhibiting mast cell activity and of abrogating the contribution of mast cells to synovial inflammation in RA.
Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative.[Pubmed:8548747]
Cancer Res. 1996 Jan 1;56(1):100-4.
Oncogenic activation of Abl proteins due to structural modifications can occur as a result of viral transduction or chromosomal translocation. The tyrosine protein kinase activity of oncogenic Abl proteins is known to be essential for their transforming activity. Therefore, we have attempted to identify selective inhibitors of the Abl tyrosine protein kinase. Herein we describe an inhibitor (CGP 57148) of the Abl and platelet-derived growth factor (PDGF) receptor protein-tyrosine kinases from the 2-phenylaminopyrimidine class, which is highly active in vitro and in vivo. Submicromolar concentrations of the compound inhibited both v-Abl and PDGF receptor autophosphorylation and PDGF-induced c-fos mRNA expression selectively in intact cells. In contrast, ligand-induced growth factor receptor autophosphorylation in response to epidermal growth factor (EGF), insulin-like growth factor-I, and insulin showed no or weak inhibition by high concentrations of CGP 57148. c-fos mRNA expression induced by EGF, fibroblast growth factor, or phorbol ester was also insensitive to inhibition by CGP 57148. In antiproliferative assays, the compound was more than 30-100-fold more potent in inhibiting growth of v-abl-transformed PB-3c cells and v-sis-transformed BALB/c 3T3 cells relative to inhibition of EGF-dependent BALB/MK cells, interleukin-3-dependent FDC-P1 cells, and the T24 bladder carcinoma line. Furthermore, anchorage-independent growth of v-abl- and v-sis-transformed BALB/c 3T3 cells was inhibited potently by CGP 57148. When tested in vivo, CGP 57148 showed antitumor activity at tolerated doses against tumorigenic v-abl- and v-sis-transformed BALB/c 3T3 cells. In contrast, CGP 57148 had no antitumor activity when tested using src-transformed BALB/c 3T3 cells. These findings suggest that CGP 57148 may have therapeutic potential for the treatment of diseases that involve abnormal cellular proliferation induced by Abl protein-tyrosine kinase deregulation or PDGF receptor activation.


