Ponatinib (AP24534)pan-BCR-ABL inhibitor,multi-kinase inhibitor CAS# 943319-70-8 |
- DCC-2036 (Rebastinib)
Catalog No.:BCC4390
CAS No.:1020172-07-9
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- PD173955
Catalog No.:BCC3999
CAS No.:260415-63-2
- Nilotinib(AMN-107)
Catalog No.:BCC3643
CAS No.:641571-10-0
- GNF 5
Catalog No.:BCC3892
CAS No.:778277-15-9
- Bafetinib (INNO-406)
Catalog No.:BCC4036
CAS No.:887650-05-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 943319-70-8 | SDF | Download SDF |
| PubChem ID | 24826799 | Appearance | Powder |
| Formula | C29H27F3N6O | M.Wt | 532.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ponatinib | ||
| Solubility | DMSO : ≥ 50 mg/mL (93.89 mM) *"≥" means soluble, but saturation unknown. | ||
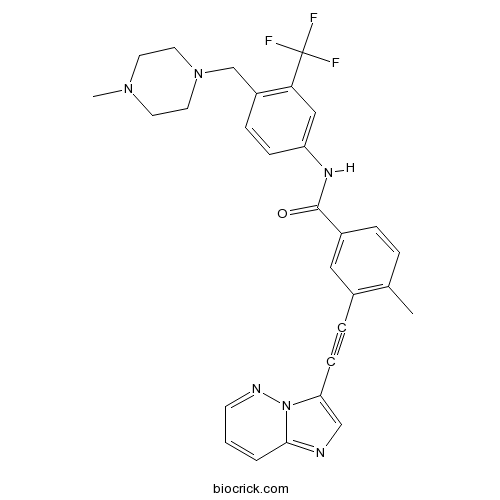
| Chemical Name | 3-(2-imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-[4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]benzamide | ||
| SMILES | CC1=C(C=C(C=C1)C(=O)NC2=CC(=C(C=C2)CN3CCN(CC3)C)C(F)(F)F)C#CC4=CN=C5N4N=CC=C5 | ||
| Standard InChIKey | PHXJVRSECIGDHY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent multi-kinase and pan-Bcr-Abl inhibitor. Displays potent activity against cell lines expressing native Bcr-Abl or Bcr-AblT3151 (IC50 values are 0.37 and 2.0 nM respectively); also inhibits other Abl kinase domain mutants at nanomolar potencies. Exhibits inhibitory activity against PDGFRα, c-Src and c-Kit (IC50 values are 1.1, 5.4 and 12.5 nM respectively); potently inhibits FGFR and VEGFR family kinases. Orally active. |

Ponatinib (AP24534) Dilution Calculator

Ponatinib (AP24534) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8777 mL | 9.3886 mL | 18.7772 mL | 37.5545 mL | 46.9431 mL |
| 5 mM | 0.3755 mL | 1.8777 mL | 3.7554 mL | 7.5109 mL | 9.3886 mL |
| 10 mM | 0.1878 mL | 0.9389 mL | 1.8777 mL | 3.7554 mL | 4.6943 mL |
| 50 mM | 0.0376 mL | 0.1878 mL | 0.3755 mL | 0.7511 mL | 0.9389 mL |
| 100 mM | 0.0188 mL | 0.0939 mL | 0.1878 mL | 0.3755 mL | 0.4694 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BCR-ABL fusion gene forms when the ABL gene from chromosome 9 joins to the BCR gene on chromosome 22. BCR-ABL is translated into a constitutively active tyrosine kinase, which is oncogenic. Depending on the fusion location, multiple protein variants are formed with molecular weight ranging from 185 to 210 kDa. BCR-ABL activates JAK/STAT pathway and MAPK signaling. [3] This gene is found in most patients with chronic myelogenous leukemia (CML), and in some patients with acute lymphoblastic leukemia (ALL) or acute myelogenous leukemia (AML).
Ponatinib is the second-generation pan inhibitor of BCR-Abl kinases, which is also effective against the mutant form of BCR-Abl (T315I). [1, 2] IC50 for WT and mutant form are 0.5 and 11 nM. [4] Ponatinib also inhibits several other clinically relevant kinases (RET, FLT3, KIT, PDGFRα, PDGFRβ, and FGFR1) in vitro, with IC50s of 5, 25, 100, 5, 9, and 23) in Ba/F3 cells lines. [4]
References:
1. Huang WS, Metcalf CA, Sundaramoorthi R, Wang Y, Zou D, Thomas RM, Zhu X, Cai L, Wen D, Liu S, Romero J, Qi J, Chen I, Banda G, Lentini SP, Das S, Xu Q, Keats J, Wang F, Wardwell S, Ning Y, Snodgrass JT, Broudy MI, Russian K, Zhou T, Commodore L, Narasimhan NI, Mohemmad QK, Iuliucci J, Rivera VM, Dalgarno DC, Sawyer TK, Clackson T, Shakespeare WC (June 2010). Discovery of 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl) methyl]-3-(trifluoromethyl)phenyl}benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant. J. Med. Chem. 53 (12): 4701–19.
2. O'Hare T, Pollock R, Stoffregen EP, Keats JA, Abdullah OM, Moseson EM, Rivera VM, Tang H, Metcalf Ca CA, Bohacek RS, Wang Y, Sundaramoorthi R, Shakespeare WC, Dalgarno D, Clackson T, Sawyer TK, Deininger MW, Druker BJ (2004). Inhibition of wild-type and mutant Bcr-Abl by AP23464, a potent ATP-based oncogenic protein kinase inhibitor: implications for CML. Blood 104 (8): 2532–2539.
3. Cilloni D and Saglio G. Molecular pathways: BCR-ABL. Clinical Cancer Res (2011) 18(4):930-937
4. Gozgit JM, Wong MJ, Zhu X, Schrock AB, Chen T, Clackson T and Rivera VM. Ponatinib, a potent pan-BCR-ABL inhibitor, retains activity against gatekeeper mutants of FLT3, RET, KIT, PDGFR α/β and FGFR1. 2012 AACR poster.
- Peroxydehydrotumulosic acid
Catalog No.:BCN3739
CAS No.:943225-53-4
- Chlorahololide D
Catalog No.:BCN4493
CAS No.:943136-39-8
- GAP-134
Catalog No.:BCC1588
CAS No.:943134-39-2
- GAP-134 Hydrochloride
Catalog No.:BCC1589
CAS No.:943133-81-1
- H-Phe(4-NH2)-OH
Catalog No.:BCC3152
CAS No.:943-80-6
- DGAT-1 inhibitor 2
Catalog No.:BCC1530
CAS No.:942999-61-3
- 3beta-(2-O-alpha-L-Rhamnopyranosyl-beta-D-xylopyranosyloxy)-23-hydroxyoleana-12-ene-28-oic acid 6-O-beta-D-glucopyranosyl-beta-D-glucopyranosyl ester
Catalog No.:BCC8948
CAS No.:942997-00-4
- 12-Hydroxyganoderic acid D
Catalog No.:BCN8051
CAS No.:942950-96-1
- CCT129202
Catalog No.:BCC2187
CAS No.:942947-93-5
- GSK1070916
Catalog No.:BCC2183
CAS No.:942918-07-2
- Acetyldihydromicromelin A
Catalog No.:BCN4492
CAS No.:94285-22-0
- Dihydromicromelin B
Catalog No.:BCN4491
CAS No.:94285-06-0
- Ethyl p-nitrobenzyl carbonate
Catalog No.:BCN3285
CAS No.:943409-69-6
- Sappanchalcone
Catalog No.:BCN4494
CAS No.:94344-54-4
- JNJ-38877605
Catalog No.:BCC2485
CAS No.:943540-75-8
- Rhapontigenin 3'-O-glucoside
Catalog No.:BCN2873
CAS No.:94356-22-6
- Piceatannol 3'-O-glucoside
Catalog No.:BCN2874
CAS No.:94356-26-0
- Methylnissolin-3-O-glucoside
Catalog No.:BCN3829
CAS No.:94367-42-7
- Isomucronulatol 7-O-glucoside
Catalog No.:BCN3845
CAS No.:94367-43-8
- Sebiferenic acid
Catalog No.:BCN4495
CAS No.:94390-09-7
- Ophiopogoside A
Catalog No.:BCC8346
CAS No.:943914-99-6
- BMS-303141
Catalog No.:BCC4097
CAS No.:943962-47-8
- Dimethylmatairesinol
Catalog No.:BCN4496
CAS No.:943989-68-2
- Tetrachlorohydroquinone dimethyl ether
Catalog No.:BCN1301
CAS No.:944-78-5
[Is AP24534 (Ponatinib) the next treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia?].[Pubmed:21700550]
Bull Cancer. 2011 Jul;98(7):761-7.
Distinct clinicopathologic acute lymphoblastic leukemia (ALL) entities have been identified, resulting in the adoption of risk-oriented treatment approaches. In Philadelphia chromosome-positive (Ph(+)) ALL, the optimal treatment requires the addition of BCR-ABL tyrosine kinase inhibitors, as imatinib. However, the outcome remains poor in absence of allogeneic stem cell transplantation, and novel agents are desperately required. Resistance attributable to kinase domain mutations can lead to relapse despite the development of second-generation compounds, including dasatinib and nilotinib. Despite these therapeutic options, the cross-resistant BCR-ABL (T315I) mutation remains a major clinical challenge. The first evaluations of AP24534 present this drug as a potent multi-targeted kinase inhibitor active against T315I and all other BCR-ABL mutants. AP24534 could be the next treatment of choice in hematological malignancies with Philadelphia-positive chromosome, particularly Ph(+) ALL known for its frequent occurrence of T315I mutation.
Ponatinib (AP24534) is a novel potent inhibitor of oncogenic RET mutants associated with thyroid cancer.[Pubmed:23526464]
J Clin Endocrinol Metab. 2013 May;98(5):E811-9.
CONTEXT: The RET tyrosine kinase encoding gene acts as a dominantly transforming oncogene in thyroid carcinoma and other malignancies. Ponatinib (AP24534) is an oral ATP-competitive tyrosine kinase inhibitor that is in advanced clinical experimentation in leukemia. OBJECTIVE: We tested whether ponatinib inhibited RET kinase and oncogenic activity. METHODS: Ponatinib activity was studied by an in vitro RET immunocomplex kinase assay and immunoblotting. The effects of ponatinib on proliferation of human TT, MZ-CRC-1, and TPC-1 thyroid carcinoma cells, which harbor endogenous oncogenic RET alleles, and of NIH3T3 fibroblasts transfected with oncogenic RET mutants were determined. Ponatinib activity on TT cell xenografted tumors in athymic mice was measured. RESULTS: Ponatinib inhibited immunopurified RET kinase at the IC(5)(0) of 25.8 nM (95% confidence interval [CI] = 23.15-28.77 nM). It also inhibited (IC(5)(0) = 33.9 nM; 95% CI = 26.41-43.58 nM) kinase activity of RET/V804M, a RET mutant displaying resistance to other tyrosine kinase inhibitor. Ponatinib blunted phosphorylation of point-mutant and rearranged RET-derived oncoproteins and inhibited proliferation of RET-transformed fibroblasts and RET mutant thyroid carcinoma cells. Finally, after 3 weeks of treatment with ponatinib (30 mg/kg/d), the volume of TT cell (medullary thyroid carcinoma) xenografts was reduced from 133 mm(3) to an unmeasurable size (difference = 133 mm(3), 95% CI = -83 to 349 mm(3)) (P < .001). Ponatinib-treated TT cell tumors displayed a reduction in the mitotic index, RET phosphorylation, and signaling. CONCLUSIONS: Ponatinib is a potent inhibitor of RET kinase and has promising preclinical activity in models of RET-driven medullary thyroid carcinoma.
Targeting wild-type and mutationally activated FGFR4 in rhabdomyosarcoma with the inhibitor ponatinib (AP24534).[Pubmed:24124571]
PLoS One. 2013 Oct 4;8(10):e76551.
Rhabdomyosarcoma (RMS) is the most common childhood soft tissue sarcoma. Despite advances in modern therapy, patients with relapsed or metastatic disease have a very poor clinical prognosis. Fibroblast Growth Factor Receptor 4 (FGFR4) is a cell surface tyrosine kinase receptor that is involved in normal myogenesis and muscle regeneration, but not commonly expressed in differentiated muscle tissues. Amplification and mutational activation of FGFR4 has been reported in RMS and promotes tumor progression. Therefore, FGFR4 is a tractable therapeutic target for patients with RMS. In this study, we used a chimeric Ba/F3 TEL-FGFR4 construct to test five tyrosine kinase inhibitors reported to specifically inhibit FGFRs in the nanomolar range. We found Ponatinib (AP24534) to be the most potent FGFR4 inhibitor with an IC50 in the nanomolar range. Ponatinib inhibited the growth of RMS cells expressing wild-type or mutated FGFR4 through increased apoptosis. Phosphorylation of wild-type and mutated FGFR4 as well as its downstream target STAT3 was also suppressed by ponatinib. Finally, ponatinib treatment inhibited tumor growth in a RMS mouse model expressing mutated FGFR4. Therefore, our data suggests that ponatinib is a potentially effective therapeutic agent for RMS tumors that are driven by a dysregulated FGFR4 signaling pathway.
Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models.[Pubmed:22238366]
Mol Cancer Ther. 2012 Mar;11(3):690-9.
Members of the fibroblast growth factor receptor family of kinases (FGFR1-4) are dysregulated in multiple cancers. Ponatinib (AP24534) is an oral multitargeted tyrosine kinase inhibitor being explored in a pivotal phase II trial in patients with chronic myelogenous leukemia due to its potent activity against BCR-ABL. Ponatinib has also been shown to inhibit the in vitro kinase activity of all four FGFRs, prompting us to examine its potential as an FGFR inhibitor. In Ba/F3 cells engineered to express activated FGFR1-4, ponatinib potently inhibited FGFR-mediated signaling and viability with IC(50) values <40 nmol/L, with substantial selectivity over parental Ba/F3 cells. In a panel of 14 cell lines representing multiple tumor types (endometrial, bladder, gastric, breast, lung, and colon) and containing FGFRs dysregulated by a variety of mechanisms, ponatinib inhibited FGFR-mediated signaling with IC(50) values <40 nmol/L and inhibited cell growth with GI(50) (concentration needed to reduce the growth of treated cells to half that of untreated cells) values of 7 to 181 nmol/L. Daily oral dosing of ponatinib (10-30 mg/kg) to mice reduced tumor growth and inhibited signaling in all three tumor models examined. Importantly, the potency of ponatinib in these models is similar to that previously observed in BCR-ABL-driven models and plasma levels of ponatinib that exceed the IC(50) values for FGFR1-4 inhibition can be sustained in patients. These results show that ponatinib is a potent pan-FGFR inhibitor and provide strong rationale for its evaluation in patients with FGFR-driven cancers.
Potent activity of ponatinib (AP24534) in models of FLT3-driven acute myeloid leukemia and other hematologic malignancies.[Pubmed:21482694]
Mol Cancer Ther. 2011 Jun;10(6):1028-35.
Ponatinib (AP24534) is a novel multitargeted kinase inhibitor that potently inhibits native and mutant BCR-ABL at clinically achievable drug levels. Ponatinib also has in vitro inhibitory activity against a discrete set of kinases implicated in the pathogenesis of other hematologic malignancies, including FLT3, KIT, fibroblast growth factor receptor 1 (FGFR1), and platelet derived growth factor receptor alpha (PDGFRalpha). Here, using leukemic cell lines containing activated forms of each of these receptors, we show that ponatinib potently inhibits receptor phosphorylation and cellular proliferation with IC50 values comparable to those required for inhibition of BCR-ABL (0.3 to 20 nmol/L). The activity of ponatinib against the FLT3-ITD mutant, found in up to 30% of acute myeloid leukemia (AML) patients, was particularly notable. In MV4-11 (FLT3-ITD(+/+)) but not RS4;11 (FLT3-ITD(-/-)) AML cells, ponatinib inhibited FLT3 signaling and induced apoptosis at concentrations of less than 10 nmol/L. In an MV4-11 mouse xenograft model, once daily oral dosing of ponatinib led to a dose-dependent inhibition of signaling and tumor regression. Ponatinib inhibited viability of primary leukemic blasts from a FLT3-ITD positive AML patient (IC50 4 nmol/L) but not those isolated from 3 patients with AML expressing native FLT3. Overall, these results support the investigation of ponatinib in patients with FLT3-ITD-driven AML and other hematologic malignancies driven by KIT, FGFR1, or PDGFRalpha.
Discovery of 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-y l)methyl]-3-(trifluoromethyl)phenyl}benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant.[Pubmed:20513156]
J Med Chem. 2010 Jun 24;53(12):4701-19.
In the treatment of chronic myeloid leukemia (CML) with BCR-ABL kinase inhibitors, the T315I gatekeeper mutant has emerged as resistant to all currently approved agents. This report describes the structure-guided design of a novel series of potent pan-inhibitors of BCR-ABL, including the T315I mutation. A key structural feature is the carbon-carbon triple bond linker which skirts the increased bulk of Ile315 side chain. Extensive SAR studies led to the discovery of development candidate 20g (AP24534), which inhibited the kinase activity of both native BCR-ABL and the T315I mutant with low nM IC(50)s, and potently inhibited proliferation of corresponding Ba/F3-derived cell lines. Daily oral administration of 20g significantly prolonged survival of mice injected intravenously with BCR-ABL(T315I) expressing Ba/F3 cells. These data, coupled with a favorable ADME profile, support the potential of 20g to be an effective treatment for CML, including patients refractory to all currently approved therapies.
AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance.[Pubmed:19878872]
Cancer Cell. 2009 Nov 6;16(5):401-12.
Inhibition of BCR-ABL by imatinib induces durable responses in many patients with chronic myeloid leukemia (CML), but resistance attributable to kinase domain mutations can lead to relapse and a switch to second-line therapy with nilotinib or dasatinib. Despite three approved therapeutic options, the cross-resistant BCR-ABL(T315I) mutation and compound mutants selected on sequential inhibitor therapy remain major clinical challenges. We report design and preclinical evaluation of AP24534, a potent, orally available multitargeted kinase inhibitor active against T315I and other BCR-ABL mutants. AP24534 inhibited all tested BCR-ABL mutants in cellular and biochemical assays, suppressed BCR-ABL(T315I)-driven tumor growth in mice, and completely abrogated resistance in cell-based mutagenesis screens. Our work supports clinical evaluation of AP24534 as a pan-BCR-ABL inhibitor for treatment of CML.


