SappanchalconeCAS# 94344-54-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 94344-54-4 | SDF | Download SDF |
| PubChem ID | 5319493 | Appearance | Orange powder |
| Formula | C16H14O5 | M.Wt | 286.3 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
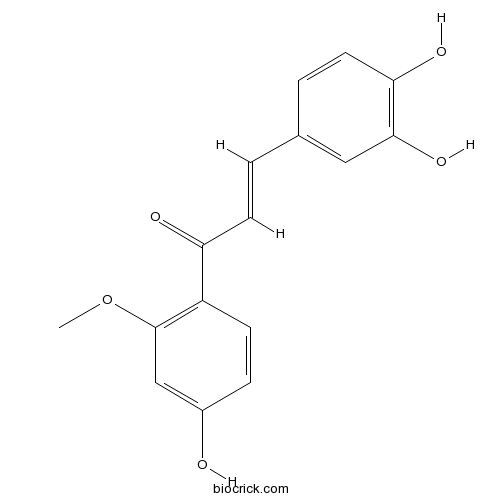
| Chemical Name | (E)-3-(3,4-dihydroxyphenyl)-1-(4-hydroxy-2-methoxyphenyl)prop-2-en-1-one | ||
| SMILES | COC1=C(C=CC(=C1)O)C(=O)C=CC2=CC(=C(C=C2)O)O | ||
| Standard InChIKey | JVGNTXGHBHMJDO-QHHAFSJGSA-N | ||
| Standard InChI | InChI=1S/C16H14O5/c1-21-16-9-11(17)4-5-12(16)13(18)6-2-10-3-7-14(19)15(20)8-10/h2-9,17,19-20H,1H3/b6-2+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Sappanchalcone has cytoprotective effect. 2. Sappanchalcone blocks cell cycle progression in the G2/M phase, brazilin inhibited TNFα/NF-κB signaling. 3. Sappanchalcone has anti-inflammatory effect, could be used as an anti-inflammatory and bone-protective agent during the treatment of rheumatoid arthritis. 4. Sappanchalcone suppresses oral cancer cell growth and induces apoptosis through the activation of p53-dependent mitochondrial, p38, ERK, JNK, and NF-κB signaling, could potentially used to treat periodontal, pulpal , periapical inflammatory lesion and oral cancer. 5. Sappanchalcone possesses the most potent effect against allergic reaction in basophilic leukemic (RBL-2H3) cells with an inhibitory concentration (IC50) value of 7.6 uM, it may have anti-allergic activity. 6. Sappanchalcone shows xanthine oxidase inhibitory activity, is a xanthine oxidase inhibitor. |
| Targets | TNF-α | IL Receptor | Bcl-2/Bax | Caspase | p53 | NF-kB | ERK | JNK | p38MAPK | HO-1 | Nrf2 | NO | PGE | STAT | NOS |

Sappanchalcone Dilution Calculator

Sappanchalcone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4928 mL | 17.4642 mL | 34.9284 mL | 69.8568 mL | 87.321 mL |
| 5 mM | 0.6986 mL | 3.4928 mL | 6.9857 mL | 13.9714 mL | 17.4642 mL |
| 10 mM | 0.3493 mL | 1.7464 mL | 3.4928 mL | 6.9857 mL | 8.7321 mL |
| 50 mM | 0.0699 mL | 0.3493 mL | 0.6986 mL | 1.3971 mL | 1.7464 mL |
| 100 mM | 0.0349 mL | 0.1746 mL | 0.3493 mL | 0.6986 mL | 0.8732 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ethyl p-nitrobenzyl carbonate
Catalog No.:BCN3285
CAS No.:943409-69-6
- Ponatinib (AP24534)
Catalog No.:BCC2522
CAS No.:943319-70-8
- Peroxydehydrotumulosic acid
Catalog No.:BCN3739
CAS No.:943225-53-4
- Chlorahololide D
Catalog No.:BCN4493
CAS No.:943136-39-8
- GAP-134
Catalog No.:BCC1588
CAS No.:943134-39-2
- GAP-134 Hydrochloride
Catalog No.:BCC1589
CAS No.:943133-81-1
- H-Phe(4-NH2)-OH
Catalog No.:BCC3152
CAS No.:943-80-6
- DGAT-1 inhibitor 2
Catalog No.:BCC1530
CAS No.:942999-61-3
- 3beta-(2-O-alpha-L-Rhamnopyranosyl-beta-D-xylopyranosyloxy)-23-hydroxyoleana-12-ene-28-oic acid 6-O-beta-D-glucopyranosyl-beta-D-glucopyranosyl ester
Catalog No.:BCC8948
CAS No.:942997-00-4
- 12-Hydroxyganoderic acid D
Catalog No.:BCN8051
CAS No.:942950-96-1
- CCT129202
Catalog No.:BCC2187
CAS No.:942947-93-5
- GSK1070916
Catalog No.:BCC2183
CAS No.:942918-07-2
- JNJ-38877605
Catalog No.:BCC2485
CAS No.:943540-75-8
- Rhapontigenin 3'-O-glucoside
Catalog No.:BCN2873
CAS No.:94356-22-6
- Piceatannol 3'-O-glucoside
Catalog No.:BCN2874
CAS No.:94356-26-0
- Methylnissolin-3-O-glucoside
Catalog No.:BCN3829
CAS No.:94367-42-7
- Isomucronulatol 7-O-glucoside
Catalog No.:BCN3845
CAS No.:94367-43-8
- Sebiferenic acid
Catalog No.:BCN4495
CAS No.:94390-09-7
- Ophiopogoside A
Catalog No.:BCC8346
CAS No.:943914-99-6
- BMS-303141
Catalog No.:BCC4097
CAS No.:943962-47-8
- Dimethylmatairesinol
Catalog No.:BCN4496
CAS No.:943989-68-2
- Tetrachlorohydroquinone dimethyl ether
Catalog No.:BCN1301
CAS No.:944-78-5
- Isomartynoside
Catalog No.:BCN4497
CAS No.:94410-22-7
- PG 106
Catalog No.:BCC6330
CAS No.:944111-22-2
Inhibitory activities of Lignum Sappan extractives on growth and growth-related signaling of tumor cells.[Pubmed:25156286]
Chin J Nat Med. 2014 Aug;12(8):607-12.
AIM: To investigate the active constituents of Lignum Sappan (Caesalpinia sappan L.) on growth-related signaling and cell mitosis. METHOD: The influence of the ethyl acetate (EtOAc) extract of Lignum Sappan and its constituents on growth-related signaling were evaluated by a luciferase assay in cells stably-transfected with NF-kappaB, STAT1, or STAT3 responsive luciferase reporter plasmid. The inhibitory effect on the cell cycle was determined by flow cytometric analysis. The anti-tumor activities were assessed in vitro and in vivo. RESULTS: The EtOAc extract of Lignum Sappan had inhibitory activities on growth-related signaling and cell mitosis. Three major active compounds were Sappanchalcone, brazilin, and butein. Sappanchalcone blocked cell cycle progression in the G2/M phase, brazilin inhibited TNFalpha/NF-kappaB signaling, while butein inhibited IL-6/STAT3 signaling, as well as TNFalpha/NF-kappaB signaling. The three compounds all demonstrated cytotoxic activities against human tumor cells in vitro. In a S180 tumor cell-bearing mice model, the anti-tumor efficacy of the EtOAc extract was better than the individual compounds acting alone. CONCLUSION: These results indicate that Lignum Sappan contains multiple active compounds with different antitumor activities, which act synergistically to enhance their anti-tumor effects. The EtOAc extract of Lignum Sappan may be better than individual active constituent as a novel medicine for the treatment of cancer.
Effects of sappanchalcone on the cytoprotection and anti-inflammation via heme oxygenase-1 in human pulp and periodontal ligament cells.[Pubmed:20621084]
Eur J Pharmacol. 2010 Oct 10;644(1-3):230-7.
Sappanchalcone has been demonstrated to possess several biological effects. However, the molecular mechanism underlying these effects is not fully understood. In this study, we examined the effects of Sappanchalcone on hydrogen peroxide (H(2)O(2))-induced cytotoxicity using human dental pulp (HDP) cells, and lipopolysaccharide (LPS)-induced inflammation using human periodontal ligament (HPDL) cells. Sappanchalone concentration proportionately increased heme oxygenase (HO)-1 protein expression and enzyme activity in both HDP and HPDL cells. It also protected HDP cells from H(2)O(2)-induced cytotoxicity and reactive oxygen species production. The cytoprotective effect of Sappanchalcone was nullified by HO-1 inhibitor, Tin protoporphyrin (SnPP). Sappanchalcone is seen to inhibit LPS-stimulated nitric oxide (NO), prostaglandin E(2) (PGE(2)), interlukine-1beta (IL-1beta), tumor necrosis factor-alpha (TNF-alpha), interlukine-6 (IL-6) and interlukine-12 (IL-12) release in addition to inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression in HPDL cells. SnPP, a specific inhibitor of HO-1, partly blocked Sappanchalcone mediated suppression of inflammatory mediator production, in LPS-stimulated HPDL cells. HDP and HPDL cells treated with Sappanchalcone exhibited the transient activation of c-Jun NH2-terminal kinase (JNK) and NF-E2-related factor-2 (Nrf2). The expression of HO-1 protein by Sappanchalcone was significantly reduced by pretreatment with JNK inhibitor. In conclusion, induction of HO-1 is an important cytoprotective mechanism by which Sappanchalcone protects HDP cells from H(2)O(2) and in addition it also exhibits anti-inflammatory effects in LPS-stimulated HPDL cells. Thus, Sappanchalcone could potentially be a therapeutic approach for periodontal, pulpal and periapical inflammatory lesion.
Anti-inflammatory activity of sappanchalcone isolated from Caesalpinia sappan L. in a collagen-induced arthritis mouse model.[Pubmed:25586964]
Arch Pharm Res. 2015 Jun;38(6):973-83.
Sappanchalcone, a bioactive flavonoid isolated from the heartwood of Caesalpinia sappan L. possesses anti-inflammatory effects. We studied the efficacy of Sappanchalcone in attenuating collagen-induced arthritis (CIA) in a mouse model of rheumatoid arthritis. Sappanchalcone was purified to homogeneity from the chloroform fraction of the methanolic extract of C. sappan, and identified using mass spectrometry and (1)H-nuclear magnetic resonance spectroscopy. CIA-induced male DBA/1J mice were divided into control, Sappanchalcone-treated, and methotrexate-treated groups (n = 10 per group). Paw swelling, arthritis severity, radiographic and histomorphometric changes were assessed to measure the protective role of Sappanchalcone against chronic disease progression. Sappanchalcone administration significantly reduced clinical arthritis and inflammatory edema in paws. Bone mineral density and trabecular structure were maintained in CIA mice administered Sappanchalcone. The levels of pro-inflammatory cytokines (TNF-alpha, IL-6, and 1L-1beta) were significantly lower in the serum of Sappanchalcone-treated mice as compared with the control group. Our results suggest that Sappanchalcone could be used as an anti-inflammatory and bone-protective agent during the treatment of rheumatoid arthritis.
Mechanism of sappanchalcone-induced growth inhibition and apoptosis in human oral cancer cells.[Pubmed:21963806]
Toxicol In Vitro. 2011 Dec;25(8):1782-8.
Sappanchalcone, a flavonoid extracted from Caesalpinia sappan, exhibits cytoprotective activity, but the molecular basis for the anticancer effect of Sappanchalcone has not been reported. In this study, we examined whether Sappanchalcone could inhibit the growth of human primary and metastatic oral cancer cells, and we analyzed the signaling pathway underlying the apoptotic effects of the compound in this process using 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) assays, fluorescence microscopy, flow cytometry, and Western blotting. Sappanchalcone-treated oral cancer cells showed an increased cytosolic level of cytochrome c, downregulated Bcl-2 expression, upregulated Bax and p53 expression, caspase-3 and -9 activation, and poly (ADP-ribose) polymerase cleavage. Furthermore, Sappanchalcone induced activation of p38, extracellular signal-regulated kinase (ERK), c-Jun amino-terminal kinase (JNK), and Nuclear factor k B (NF-kappaB), as demonstrated by the phosphorylation of each mitogen-activated protein kinases (MAPKs), the degradation of inhibitor of NF-kappaalpha (IkappaB-alpha), increased expression of nuclear p65, and NF-kappaB-DNA binding. Inhibition of the expression of p38, ERK, JNK, and NF-kappaB by pharmacological inhibitors reversed Sappanchalcone-induced growth inhibition and apoptosis. These results provide the first evidence that Sappanchalcone suppresses oral cancer cell growth and induces apoptosis through the activation of p53-dependent mitochondrial, p38, ERK, JNK, and NF-kappaB signaling. Thus, it has potential as a chemotherapeutic agent for oral cancer.


