CCT129202Aurora kinase inhibitor,ATP-competitive and potent CAS# 942947-93-5 |
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- AT7867 dihydrochloride
Catalog No.:BCC1378
CAS No.:1431697-86-7
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- A-674563
Catalog No.:BCC3903
CAS No.:552325-73-2
- AT7867
Catalog No.:BCC2536
CAS No.:857531-00-1
- 10-DEBC hydrochloride
Catalog No.:BCC7409
CAS No.:925681-41-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 942947-93-5 | SDF | Download SDF |
| PubChem ID | 16202152 | Appearance | Powder |
| Formula | C23H25ClN8OS | M.Wt | 497.02 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 1 mg/mL (2.01 mM; Need ultrasonic) | ||
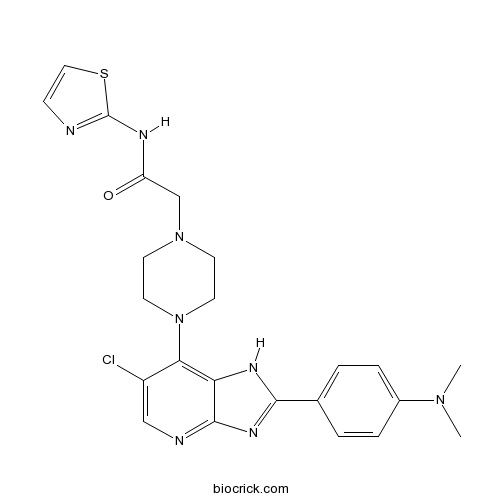
| Chemical Name | 2-[4-[6-chloro-2-[4-(dimethylamino)phenyl]-1H-imidazo[4,5-b]pyridin-7-yl]piperazin-1-yl]-N-(1,3-thiazol-2-yl)acetamide | ||
| SMILES | CN(C)C1=CC=C(C=C1)C2=NC3=NC=C(C(=C3N2)N4CCN(CC4)CC(=O)NC5=NC=CS5)Cl | ||
| Standard InChIKey | QYKHWEFPFAGNEV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H25ClN8OS/c1-30(2)16-5-3-15(4-6-16)21-28-19-20(17(24)13-26-22(19)29-21)32-10-8-31(9-11-32)14-18(33)27-23-25-7-12-34-23/h3-7,12-13H,8-11,14H2,1-2H3,(H,25,27,33)(H,26,28,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CCT129202 is an inhibitor of Aurora kinases with IC50 values of 42 nM, 198 nM and 227 nM for Aurora A, B and C, respectively. | |||||
| Targets | Aurora A | Aurora B | Aurora C | |||
| IC50 | 42 nM | 198 nM | 227 nM | |||

CCT129202 Dilution Calculator

CCT129202 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.012 mL | 10.06 mL | 20.1199 mL | 40.2398 mL | 50.2998 mL |
| 5 mM | 0.4024 mL | 2.012 mL | 4.024 mL | 8.048 mL | 10.06 mL |
| 10 mM | 0.2012 mL | 1.006 mL | 2.012 mL | 4.024 mL | 5.03 mL |
| 50 mM | 0.0402 mL | 0.2012 mL | 0.4024 mL | 0.8048 mL | 1.006 mL |
| 100 mM | 0.0201 mL | 0.1006 mL | 0.2012 mL | 0.4024 mL | 0.503 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CCT129202, a derivative of the piperazinyl imidazo[4,5-b] pyridine scaffold, is a novel and potent inhibitor of Aurora kinase that ATP-competitively inhibits Aurora A, Aurora B and Aurora C with values of 50% inhibition concentration IC50 of 0.042, 0.198 and 0.227 μmol/L respectively. CCT129202 exhibits anti-cancer activity against multiple human tumor cell lines through inhibiting proliferation, inducing apoptosis and delaying mitosis, abrogation of nocodazole-induced mitotic arrest and spindle defects. Recent study results have shown that CCT129202 inhibits the growth of HCT116 xenografts in nude mice and induces the production of a cyclin-dependent kinase inhibitor, p21, in HCT116 cells consequently leading to Rb hypophosphorylation.
Reference
Chan F, Sun C, Perumal M, Nguyen QD, Bavetsias V, McDonald E, Martins V, Wilsher NE, Raynaud FI, Valenti M, Eccles S, Te Poele R, Workman P, Aboagye EO, Linardopoulos S. Mechanism of action of the Aurora kinase inhibitor CCT129202 and in vivo quantification of biological activity. Mol Cancer Ther. 2007; 6(12 Pt1): 3147-3157.
- GSK1070916
Catalog No.:BCC2183
CAS No.:942918-07-2
- Acetyldihydromicromelin A
Catalog No.:BCN4492
CAS No.:94285-22-0
- Dihydromicromelin B
Catalog No.:BCN4491
CAS No.:94285-06-0
- 5-Hydroxy-7,8,2',5'-tetramethoxyflavone 5-O-glucoside
Catalog No.:BCN1302
CAS No.:942626-75-7
- 1,5,15-Tri-O-methylmorindol
Catalog No.:BCN4490
CAS No.:942609-65-6
- Walsuronoid B
Catalog No.:BCN4489
CAS No.:942582-15-2
- PF-03814735
Catalog No.:BCC2184
CAS No.:942487-16-3
- Nemoralisin
Catalog No.:BCN4488
CAS No.:942480-13-9
- VKGILS-NH2
Catalog No.:BCC3953
CAS No.:942413-05-0
- Cycloart-24-ene-1alpha,2alpha,3beta-triol
Catalog No.:BCN7983
CAS No.:942407-97-8
- Scutebarbatine I
Catalog No.:BCN1026
CAS No.:960302-84-5
- 20-Dehydroeupatoriopicrin semiacetal
Catalog No.:BCN7370
CAS No.:94234-24-9
- 12-Hydroxyganoderic acid D
Catalog No.:BCN8051
CAS No.:942950-96-1
- 3beta-(2-O-alpha-L-Rhamnopyranosyl-beta-D-xylopyranosyloxy)-23-hydroxyoleana-12-ene-28-oic acid 6-O-beta-D-glucopyranosyl-beta-D-glucopyranosyl ester
Catalog No.:BCC8948
CAS No.:942997-00-4
- DGAT-1 inhibitor 2
Catalog No.:BCC1530
CAS No.:942999-61-3
- H-Phe(4-NH2)-OH
Catalog No.:BCC3152
CAS No.:943-80-6
- GAP-134 Hydrochloride
Catalog No.:BCC1589
CAS No.:943133-81-1
- GAP-134
Catalog No.:BCC1588
CAS No.:943134-39-2
- Chlorahololide D
Catalog No.:BCN4493
CAS No.:943136-39-8
- Peroxydehydrotumulosic acid
Catalog No.:BCN3739
CAS No.:943225-53-4
- Ponatinib (AP24534)
Catalog No.:BCC2522
CAS No.:943319-70-8
- Ethyl p-nitrobenzyl carbonate
Catalog No.:BCN3285
CAS No.:943409-69-6
- Sappanchalcone
Catalog No.:BCN4494
CAS No.:94344-54-4
- JNJ-38877605
Catalog No.:BCC2485
CAS No.:943540-75-8
Mechanism of action of the Aurora kinase inhibitor CCT129202 and in vivo quantification of biological activity.[Pubmed:18089709]
Mol Cancer Ther. 2007 Dec;6(12 Pt 1):3147-57.
The Aurora family of serine/threonine kinases is important for the regulation of centrosome maturation, chromosome segregation, and cytokinesis during mitosis. Overexpression of Aurora kinases in mammalian cells leads to genetic instability and transformation. Increased levels of Aurora kinases have also been linked to a broad range of human tumors. Here, we describe the properties of CCT129202, a representative of a structurally novel series of imidazopyridine small-molecule inhibitors of Aurora kinase activity. This compound showed high selectivity for the Aurora kinases over a panel of other kinases tested and inhibits proliferation in multiple cultured human tumor cell lines. CCT129202 causes the accumulation of human tumor cells with >or=4N DNA content, leading to apoptosis. CCT120202-treated human tumor cells showed a delay in mitosis, abrogation of nocodazole-induced mitotic arrest, and spindle defects. Growth of HCT116 xenografts in nude mice was inhibited after i.p. administration of CCT129202. We show that p21, the cyclin-dependent kinase inhibitor, is induced by CCT129202. Up-regulation of p21 by CCT129202 in HCT116 cells led to Rb hypophosphorylation and E2F inhibition, contributing to a decrease in thymidine kinase 1 transcription. This has facilitated the use of 3'-deoxy-3'[(18)F]fluorothymidine-positron emission tomography to measure noninvasively the biological activity of the Aurora kinase inhibitor CCT129202 in vivo.
Protein kinase C modulates Aurora-kinase inhibition induced by CCT129202 in HMC-1(5)(6)(0),(8)(1)(6) cell line.[Pubmed:23701310]
Antiinflamm Antiallergy Agents Med Chem. 2013;12(3):265-76.
The human mast cell line HMC-1(5)(6)(0),(8)(1)(6) carries activating mutations in the proto-oncogene of c-kit that cause autophosphorylation and permanent c-kit receptor activation. The compound CCT129202 is a new and selective inhibitor of Aurora kinase A and B that decreases the viability of a variety of human tumor cell lines. The effect of Aurora kinase inhibition was assessed in the HMC-1(5)(6)(0),(8)(1)(6) line in order to find a suitable tool for mastocytosis treatment. CCT129202 treatment induces a significant decrease in cell viability in HMC-1(5)(6)(0),(8)(1)(6) cells after 48 hours of treatment. Moreover, caspase-3 and caspase-8 activation was induced after incubation of HMC-1(5)(6)(0),(8)(1)(6) cells in the presence of CCT129202. It has been demonstrated that Protein Kinase C (PKC) plays a crucial role in mast cell activation as well as cell migration, adhesion and apoptotic cell death. Co-treatment of Ca(2)(+)-independent PKCs (delta epsilon and theta) inhibitor GF109203X with CCT129202, reduces caspase-3 activation which controls cell levels. In contrast, Go6976, an inhibitor of Ca(2)(+)-dependent PKCs, increases caspase-3 activation. Oppositely, GF109203X does not modify CCT129202-induced apoptosis through the caspase-8 pathway whereas Go6976 treatment abolishes the increase on caspase-8 activity due to CCT129202. This implies that Ca(2)(+)-independent PKC isoforms seems to be related with CCT129202-induced apoptosis through the caspase- 3 pathway, whereas Ca(2)(+)-dependent PKC isoforms are related with the CCT129202 effect on the caspase-8 pathway. Interestingly, CCT129202 cytotoxic effect remains even though Ca(2)(+)-dependent PKCs are inhibited, which shows that the Aurora kinase inhibitor effect is acting through the caspase-3 pathway. On the other hand, Ca(2)(+)-independent PKCs inhibition does not affect the final apoptotic CCT129202 effect because this seems to be mediated by the caspase-8 pathway. Moreover, CCT129202 does not affect PKCdelta and Ca(2)(+)-dependent PKC translocation, which indicates that PKC translocation pivots on its activation. This demonstrates that Aurora kinase inhibition is not related to this process. Finally, when PKC is silenced in HMC-1(5)(6)(0),(8)(1)(6) cells, the effect of CCT129202 on the caspase-3 pathway disappears, which indicates that the CCT129202 effect is clearly PKC-dependent.
Enhancing chemosensitivity in ABCB1- and ABCG2-overexpressing cells and cancer stem-like cells by an Aurora kinase inhibitor CCT129202.[Pubmed:22632055]
Mol Pharm. 2012 Jul 2;9(7):1971-82.
Imidazopyridine CCT129202 is an inhibitor of Aurora kinase activity and displays a favorable antineoplastic effect in preclinical studies. Here, we investigated the enhanced effect of CCT129202 on the cytotoxicity of chemotherapeutic drugs in multidrug resistant (MDR) cells with overexpression of ATP-binding cassette (ABC) transporters and cancer stem-like cells. CCT129202 of more than 90% cell survival concentration significantly enhanced the cytotoxicity of substrate drugs and increased the intracellular accumulations of doxorubicin and rhodamine 123 in ABCB1 and ABCG2 overexpressing cells, while no effect was found on parental sensitive cells. Interestingly, CCT129202 also potentiated the sensitivity of cancer stem-like cells to doxorubicin. Importantly, CCT129202 increased the inhibitory effect of vincristine and paclitaxel on ABCB1 overexpressing KBv200 cell xenografts in nude mice and human esophageal cancer tissue overexpressing ABCB1 ex vivo, respectively. Furthermore, the ATPase activity of ABCB1 was inhibited by CCT129202. Homology modeling predicted the binding conformation of CCT129202 within the large hydrophobic cavity of ABCB1. On the other hand, CCT129202 neither apparently altered the expression levels of ABCB1 and ABCG2 nor inhibited the activity of Aurora kinases in MDR cells under the concentration of reversal MDR. In conclusion, CCT129202 significantly reversed ABCB1- and ABCG2-mediated MDR in vitro, in vivo and ex vivo by inhibiting the function of their transporters and enhanced the eradication of cancer stem-like cells by chemotherapeutic agents. CCT129202 may be a candidate as MDR reversal agent for antineoplastic combination therapy and merits further clinical investigation.


