VKGILS-NH2control peptide for SLIGKV-NH2, PAR1 agonist CAS# 942413-05-0 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 942413-05-0 | SDF | Download SDF |
| PubChem ID | 90488839 | Appearance | Powder |
| Formula | C28H54N8O7 | M.Wt | 614.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in water | ||
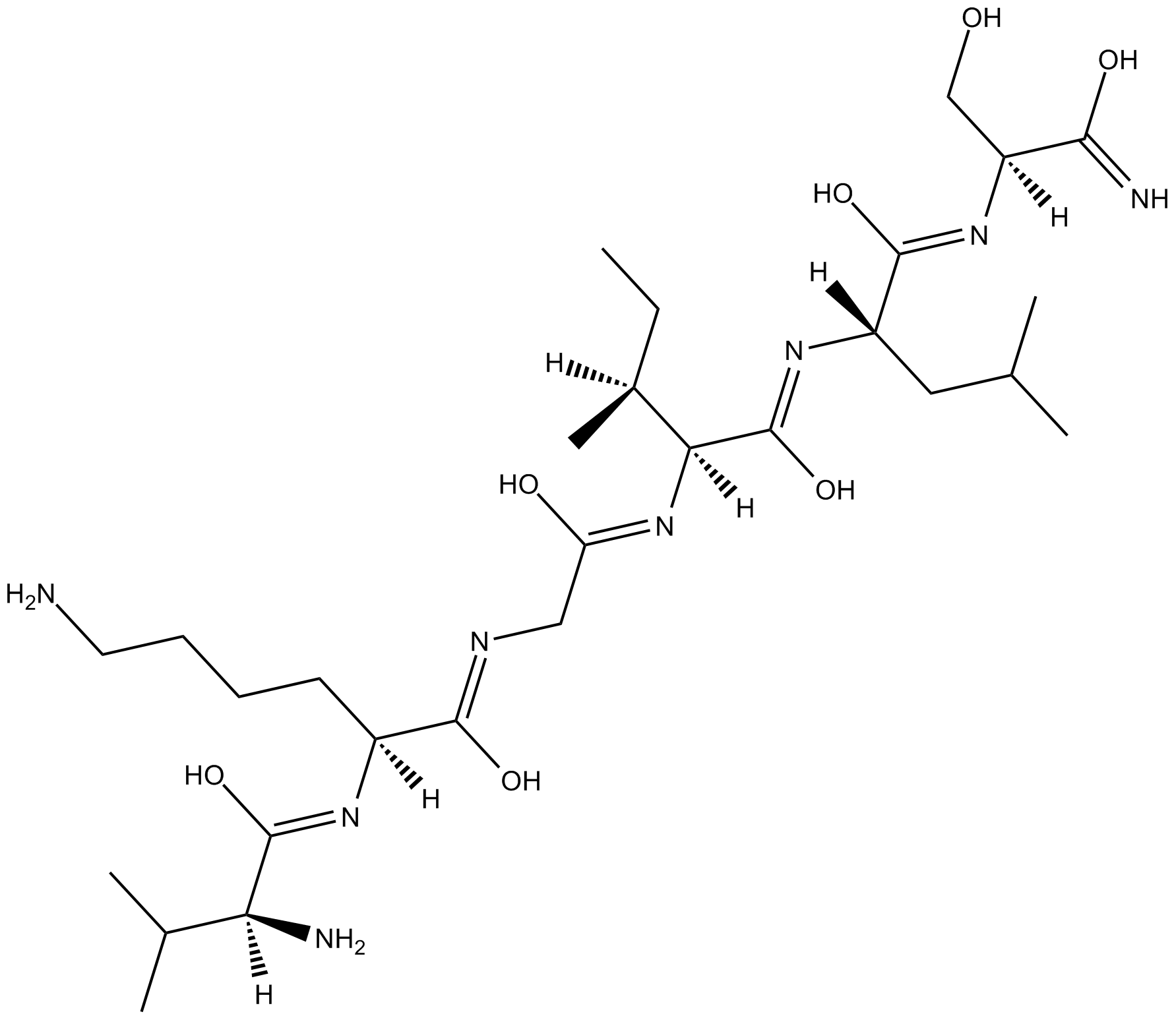
| Sequence | VKGILS (Modifications: Ser-6 = C-terminal amide) | ||
| Chemical Name | (2S)-6-amino-N-[2-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-amino-3-hydroxy-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-2-oxoethyl]-2-[[(2S)-2-amino-3-methylbutanoyl]amino]hexanamide | ||
| SMILES | CCC(C)C(C(=O)NC(CC(C)C)C(=O)NC(CO)C(=O)N)NC(=O)CNC(=O)C(CCCCN)NC(=O)C(C(C)C)N | ||
| Standard InChIKey | IYJUKRSADJIIBX-WAUHAFJUSA-N | ||
| Standard InChI | InChI=1S/C28H54N8O7/c1-7-17(6)23(28(43)34-19(12-15(2)3)26(41)35-20(14-37)24(31)39)36-21(38)13-32-25(40)18(10-8-9-11-29)33-27(42)22(30)16(4)5/h15-20,22-23,37H,7-14,29-30H2,1-6H3,(H2,31,39)(H,32,40)(H,33,42)(H,34,43)(H,35,41)(H,36,38)/t17-,18-,19-,20-,22-,23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reversed amino acid sequence control peptide for SLIGKV-NH2, a protease-activated receptor 2 (PAR2) agonist. |

VKGILS-NH2 Dilution Calculator

VKGILS-NH2 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Not Available.
VKGILS-NH2 serves as the reversed amino acid sequence control peptide for SLIGKV-NH2, a protease-activated receptor 2 (PAR2) agonist. PARs are a group of G-protein-coupled receptors existing in several cell types. Up to date, four PAR members including PAR1 to 4 have been identified, cloned and designated. PAR2 is expressed in the respiratory and gastrointestinal tracts. It is suggested that the activation of PAR2 is closely correlated with inflammatory evens in various cells and tissues. PAR2 has also been identified to induce protease activation and therefore result in systemic hypotension. [1]
In vitro: The PAR2 activating peptide AP (SLIGKV-NH2) and the reverse peptide control RP (VKGILS-NH2) were used in one study to reveal that PAR2 slightly enhanced mucin secretion by human bronchial epithelial cells in vitro. According to this study, compared to cells treated with VKGILS-NH2, exposure of cells to the synthetic PAR2 agonist peptide (SLIGKV-NH2) for 30 mins resulted in a weak but statistically significant increase in mucin secretion at concentrations of 100 and 1000M. [1]
In vivo: In one in vivo study, the mouse PAR2 reverse (LRGILS-NH2) and human PAR2 reverse (VKGILS-NH2) peptides were adopted as control which caused no contractile responses at the highest concentrations, with the aim to prove in the guinea-pig gallbladder PAR1 and PAR2 activation could lead to contractile responses. [2]
Clinical trial: PAR2 activating peptide, SLIGKV-NH2, and its reverse-sequence control peptide VKGILS-NH2 were synthesized to verify the hypothesis that in vivo activation of PAR2 in humans would cause vasodilatation. The result of this study showed that, in forearm resistance vessels, SLIGKV-NH2 triggered a dose-dependent dilatation, while VKGILS-NH2 had no significant effect. [3]
References:
[1] Lin KW, Park J, Crews AL, Li YH, Adler KB. Protease-activated receptor-2 (PAR-2) is a weak enhancer of mucin secretion by human bronchial epithelial cells in vitro. Int J Biochem Cell B. 2008. 40: 137988.
[2] Tognetto M, Trevisani M, Maggiore B, Navarra G, Turini A, Guerrini R, Bunnett NW, Geppetti P and Harrison S. Evidence that PAR-1 and PAR-2 mediate prostanoid-dependent contraction in isolated guinea-pig gallbladder. Br.J.Pharmacol. 2000.131: 689-94.
[3] Robin J, Kharbanda R, Mclean P, Campbell R, Vallance P. Protease-activated receptor 2–mediated vasodilatation in humans in vivo, role of nitric oxide and prostanoids. Circulation. 2003;107:954-959.
- Cycloart-24-ene-1alpha,2alpha,3beta-triol
Catalog No.:BCN7983
CAS No.:942407-97-8
- Scutebarbatine I
Catalog No.:BCN1026
CAS No.:960302-84-5
- 20-Dehydroeupatoriopicrin semiacetal
Catalog No.:BCN7370
CAS No.:94234-24-9
- PI 828
Catalog No.:BCC7494
CAS No.:942289-87-4
- SCH772984
Catalog No.:BCC1935
CAS No.:942183-80-4
- VTP-27999
Catalog No.:BCC2048
CAS No.:942142-51-0
- Methyl 3-indolecarboxylate
Catalog No.:BCN4487
CAS No.:942-24-5
- 8beta-(4'-Hydroxytigloyloxy)costunolide
Catalog No.:BCN7885
CAS No.:94190-32-6
- S-Ruxolitinib (INCB018424)
Catalog No.:BCC2201
CAS No.:941685-37-6
- INCB032304
Catalog No.:BCC6455
CAS No.:941685-27-4
- Ruxolitinib (INCB018424)
Catalog No.:BCC1276
CAS No.:941678-49-5
- Selaginellin
Catalog No.:BCN8215
CAS No.:941269-84-7
- Nemoralisin
Catalog No.:BCN4488
CAS No.:942480-13-9
- PF-03814735
Catalog No.:BCC2184
CAS No.:942487-16-3
- Walsuronoid B
Catalog No.:BCN4489
CAS No.:942582-15-2
- 1,5,15-Tri-O-methylmorindol
Catalog No.:BCN4490
CAS No.:942609-65-6
- 5-Hydroxy-7,8,2',5'-tetramethoxyflavone 5-O-glucoside
Catalog No.:BCN1302
CAS No.:942626-75-7
- Dihydromicromelin B
Catalog No.:BCN4491
CAS No.:94285-06-0
- Acetyldihydromicromelin A
Catalog No.:BCN4492
CAS No.:94285-22-0
- GSK1070916
Catalog No.:BCC2183
CAS No.:942918-07-2
- CCT129202
Catalog No.:BCC2187
CAS No.:942947-93-5
- 12-Hydroxyganoderic acid D
Catalog No.:BCN8051
CAS No.:942950-96-1
- 3beta-(2-O-alpha-L-Rhamnopyranosyl-beta-D-xylopyranosyloxy)-23-hydroxyoleana-12-ene-28-oic acid 6-O-beta-D-glucopyranosyl-beta-D-glucopyranosyl ester
Catalog No.:BCC8948
CAS No.:942997-00-4
- DGAT-1 inhibitor 2
Catalog No.:BCC1530
CAS No.:942999-61-3
Protease-activated receptor-2 (PAR-2) is a weak enhancer of mucin secretion by human bronchial epithelial cells in vitro.[Pubmed:18077203]
Int J Biochem Cell Biol. 2008;40(6-7):1379-88.
PAR-2, a member of a family of G-protein-coupled receptors, can be activated by serine proteases via proteolytic cleavage. PAR-2 expression is known to be upregulated in respiratory epithelium subsequent to inflammation in asthma and chronic obstructive pulmonary disease (COPD). Since these diseases also are characterized by excessive mucus production and secretion, we investigated whether PAR-2 could be linked to mucin hypersecretion by airway epithelium. Normal human bronchial epithelial (NHBE) cells in primary culture or the human bronchial epithelial cell lines, NCI-H292 and HBE-1, were used. NHBE, NCI-H292, and HBE-1 cells expressed prominent levels of PAR-2 protein. Short-term (30min) exposure of cells to the synthetic PAR-2 agonist peptide (SLIGKV-NH2) elicited a small but statistically significant increase in mucin secretion at high concentrations (100microM and 1000microM), compared to a control peptide with reversed amino acid sequence (VKGILS-NH2). Neither human lung tryptase nor bovine pancreatic trypsin, both PAR-2 agonists, affected NHBE cell mucin secretion when added over a range of concentrations. Knockdown of PAR-2 expression by siRNA blocked the stimulatory effect of the AP. The results suggest that, since PAR-2 activation only weakly increases mucin secretion by human airway epithelial cells in vitro, PAR-2 probably is not a significant contributor to mucin hypersecretion in inflamed airways.
Proinflammatory and proliferative responses of human proximal tubule cells to PAR-2 activation.[Pubmed:17699557]
Am J Physiol Renal Physiol. 2007 Nov;293(5):F1441-9.
Despite the abundant expression of protease-activated receptor (PAR)-2 in the kidney, its relevance to renal physiology is not well understood. A role for this receptor in inflammation and cell proliferation has recently been suggested in nonrenal tissues. The aims of this study were to demonstrate that human proximal tubule cells (PTC) express functional PAR-2 and to investigate whether its activation can mediate proinflammatory and proliferative responses in these cells. Primary human PTC were cultured under serum-free conditions with or without the PAR-2-activating peptide SLIGKV-NH2 (up to 800 microM), a control peptide, VKGILS-NH2 (200 microM), or trypsin (0.01-100 nM). PAR-2 expression (RT-PCR), intracellular Ca2+ mobilization (fura-2 fluorimetry), DNA synthesis (thymidine incorporation), fibronectin production (ELISA, Western blotting), and monocyte chemotactic protein (MCP)-1 secretion (ELISA) were measured. Trypsinogen expression in kidney and PTC cultures was determined by immunohistochemistry and Western blotting. In the kidney PTC were the predominant cell type expressing PAR-2. SLIGKV-NH2, but not VKGILS-NH2, stimulated a rapid concentration-dependent mobilization of intracellular Ca2+ and ERK1/2 phosphorylation and, by 24 h, increases in DNA synthesis, fibronectin secretion, and MCP-1 secretion. These delayed responses appeared to be independent of ERK1/2. Trypsin produced similar rapid but not delayed responses. Trypsinogen was weakly expressed by PTC in the kidney and in culture. In summary, PTC are the main site of PAR-2 expression in the human kidney. In PTC cultures SLIGKV-NH2 initiates proinflammatory and proliferative responses. Trypsinogen expressed within the kidney has the potential to contribute to PAR-2 activation in certain circumstances.
Activation of human colon mast cells through proteinase activated receptor-2.[Pubmed:14760751]
World J Gastroenterol. 2004 Feb 1;10(3):327-31.
AIM: To investigate the ability of agonists of PAR-2 to stimulate release of tryptase and histamine from human colon mast cells and the potential mechanisms. METHODS: Enzymatically dispersed cells from human colons were challenged with tc-LIGRLO, tc-OLRGIL, SLIGKV, VKGILS, trypsin, anti-IgE or calcium ionophore A23187, and the cell supernatants after challenge were collected. Tryptase release was determined with a sandwich ELISA procedure and histamine release was measured using a glass fibre-based fluorometric assay. RESULTS: Both PAR-2 agonists tc-LIGRLO-NH2 and SLIGKV-NH2 were able to induce dose dependent release of tryptase and histamine from colon mast cells. More than 2.5 fold increase in both tryptase and histamine release was provoked by 100 micromol/mL tc-LIGRLO-NH2, in comparison with only 2.0 fold increase being stimulated by SLIGKV-NH2. The reverse peptides tc-OLRGIL-NH2 and VKGILS -NH2 at the concentrations tested had no effect on the release of these two mediators. The maximum tryptase release elicited by tc-LIGRLO-NH2 was similar to that induced by anti-IgE (10 microg/mL) or calcium ionophore (1 microg/mL), though the latter was a more potent stimulus for histamine release. Both histamine and tryptase release in response to tc-LIGRLO-NH2 were completed within 3 min. Trypsin at concentrations from 1.0 to 100 microg/mL was capable of provoking a dose dependent release of tryptase as well as histamine with a maximum of 16 ng/mL tryptase and 14 ng/mL histamine release being achieved. An approximately 80% and 70% inhibition of trypsin induced release of tryptase and histamine were observed with SBTI, respectively. Pretreatment of cells with metabolic inhibitors or pertussis toxin abolished the actions of tc-LIGRLO-NH2, SLIGKV-NH2 and trypsin. CONCLUSION: The agonists of PAR-2 and trypsin are potent secretagogues of human colon mast cells, which are likely to contribute to the development of inflammatory disorders in human gut.
Protease-activated receptor-1 (PAR1) and PAR2 but not PAR4 mediate relaxations in lower esophageal sphincter.[Pubmed:17335921]
Regul Pept. 2007 Jul 5;142(1-2):37-43.
Protease-activated receptor-1 (PAR1), PAR2 and PAR4 activation can alter the gastrointestinal motility. To investigate effects mediated by PARs in the lower esophageal sphincter, we measured contraction or relaxation of transverse strips from the guinea-pig lower esophageal sphincter caused by PAR1 (TFLLR-NH2 and SFLLRN-NH2), PAR2 (SLIGKV-NH2 and SLIGRL-NH2) and PAR4 peptide agonists (GYPGKF-NH2, GYPGQV-NH2 and AYPGKF-NH2) as well as PAR protease activators (thrombin and trypsin). In resting lower esophageal sphincter strips, TFLLR-NH2 and SFLLRN-NH2 caused moderate concentration-dependent relaxation whereas thrombin did not cause any relaxation or contraction. Furthermore, in carbachol-contracted strips, TFLLR-NH2 and SFLLRN-NH2 caused marked whereas thrombin caused mild concentration-dependent relaxation. These indicate the existence of PAR1 mediating relaxation. Similarly, in resting lower esophageal sphincter strips, trypsin caused moderate concentration-dependent relaxation whereas SLIGRL-NH2 and SLIGKV-NH2 did not cause any relaxation or contraction. In addition, in carbachol-contracted strips, trypsin caused marked whereas SLIGRL-NH2 and SLIGKV-NH2 caused mild concentration-dependent relaxation. These indicate the existence of PAR2 mediating relaxation. The relaxant response of thrombin, TFLLR-NH2, trypsin and SLIGKV-NH2 was insensitive to atropine or tetrodotoxin, suggesting a direct effect. The relaxant response of trypsin was not affected by apamin, charybdotoxin, indomethacin and capsaicin but was attenuated by NG-nitro-L-arginine methyl ester, indicating involvement of NO. FSLLR-NH2, a PAR1 control peptide, and VKGILS-NH2, a PAR2 control peptide, as well as all three PAR4 peptide agonists, GYPGKF-NH2, GYPGQV-NH2 and AYPGKF-NH2, did not cause any relaxation or contraction. Taken together, these results demonstrate that PAR1 and PAR2 but not PAR4 mediate relaxations in the guinea-pig lower esophageal sphincter.
Proteinase-activated receptor-1 (PAR(1)) and PAR(2) but not PAR(4) mediate contraction in human and guinea-pig gallbladders.[Pubmed:18179608]
Neurogastroenterol Motil. 2008 Apr;20(4):385-91.
Proteinase-activated receptor-1 (PAR(1)) and PAR(2) mediate contraction in the guinea-pig gallbladder. To investigate and compare the effects mediated by PARs in the human gallbladder with those in the guinea-pig gallbladder, we measured contractions of isolated human and guinea-pig gallbladder strips caused by PAR agonists. Results in human were similar to those in guinea-pig gallbladder. The PAR(1) agonists, thrombin, TFLLR-NH2 and SFLLRN-NH2, as well as the PAR(2) agonists, trypsin, SLIGKV-NH2 and SLIGRL-NH2, caused contraction in both human and guinea-pig gallbladders. These indicate the existence of PAR(1) and PAR(2) mediating gallbladder contraction. Furthermore, the existence of PAR(1) and PAR(2) in the human gallbladder was confirmed by reverse transcription-polymerase chain reaction. In contrast, FSLLR-NH2, a PAR(1) control peptide, and VKGILS-NH2, a PAR(2) control peptide, as well as three PAR(4) agonists, GYPGKF-NH2, GYPGQV-NH2 and AYPGKF-NH2, did not cause any contraction or relaxation. The contractile responses to TFLLR-NH2, SFLLRN-NH2 and trypsin in both human and guinea-pig gallbladders were insensitive to atropine and tetrodotoxin, suggesting direct effects. These results demonstrate that, similar to the guinea-pig gallbladder, both PAR(1) and PAR(2) but not PAR(4) mediate muscle contraction in the human gallbladder. PAR(1) and PAR(2) may play important roles in the control of both human and guinea-pig gallbladder motility.
Evidence that PAR-1 and PAR-2 mediate prostanoid-dependent contraction in isolated guinea-pig gallbladder.[Pubmed:11030717]
Br J Pharmacol. 2000 Oct;131(4):689-94.
We have investigated the ability of protease-activated receptor-1 (PAR-1), PAR-2, PAR-3 and PAR-4 agonists to induce contractile responses in isolated guinea-pig gallbladder. Thrombin, trypsin, mouse PAR-1 activating (SFLLRN-NH(2)) peptide, and mouse PAR-2 activating (SLIGRL-NH(2)) and human PAR-2 activating (SLIGKV-NH(2)) peptides produced a concentration-dependent contractile response. Mouse PAR-4 activating (GYPGKF-NH(2)) peptide, the mouse PAR-1 reverse (NRLLFS-NH(2)) peptide, the mouse PAR-2 reverse (LRGILS-NH(2)) and human PAR-2 reverse (VKGILS-NH(2)) peptides caused negligible contractile responses at the highest concentrations tested. An additive effect was observed following the contractile response induced by either trypsin or thrombin, with the addition of a different PAR agonist (SFLLRN-NH(2) and SLIGRL-NH(2), respectively). Desensitization to PAR-2 activating peptide attenuated the response to trypsin but failed to attenuate the response to PAR-1 agonists, and conversely desensitization to PAR-1 attenuated the response to thrombin but failed to alter contractile responses to PAR-2 agonists. The contractile responses produced by thrombin, trypsin, SFLLRN-NH(2) and SLIGRL-NH(2) were markedly reduced in the presence of the cyclo-oxygenase inhibitor, indomethacin, whilst the small contractile response produced by NRLLFS-NH(2) and LRGILS-NH(2) were insensitive to indomethacin. The contractile responses to thrombin, trypsin, SFLLRN-NH(2) and SLIGRL-NH(2) were unaffected by the presence of: the non-selective muscarinic antagonist, atropine; the nitric oxide synthase inhibitor, L-NAME; the sodium channel blocker, tetrodotoxin; the combination of selective tachykinin NK(1) and NK(2) receptor antagonists, (S)-1-[2-[3-(3,4-dichlorphenyl)-1 (3-isopropoxyphenylacetyl) piperidin-3-yl] ethyl]-4-phenyl-1 azaniabicyclo [2.2.2] octane chloride (SR140333) and (S)-N-methyl-N-[4-acetylamino-4-phenylpiperidino-2-(3, 4-dichlorophenyl)-butyl] benzamide (SR48968), respectively. The results indicate that PAR-1 and PAR-2 activation causes contractile responses in the guinea-pig gallbladder, an effect that is mediated principally by prostanoid release, and is independent of neural mechanisms.


