PF-03814735Aurora A/B inhibitor CAS# 942487-16-3 |
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- Istaroxime
Catalog No.:BCC1660
CAS No.:203737-93-3
- Dynasore
Catalog No.:BCC1088
CAS No.:304448-55-3
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 942487-16-3 | SDF | Download SDF |
| PubChem ID | 51346455 | Appearance | Powder |
| Formula | C23H25F3N6O2 | M.Wt | 474.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (210.76 mM) *"≥" means soluble, but saturation unknown. | ||
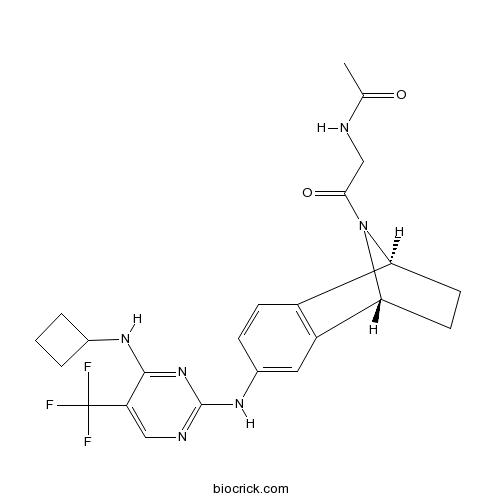
| Chemical Name | N-[2-[(1R,8S)-4-[[4-(cyclobutylamino)-5-(trifluoromethyl)pyrimidin-2-yl]amino]-11-azatricyclo[6.2.1.02,7]undeca-2(7),3,5-trien-11-yl]-2-oxoethyl]acetamide | ||
| SMILES | CC(=O)NCC(=O)N1C2CCC1C3=C2C=CC(=C3)NC4=NC=C(C(=N4)NC5CCC5)C(F)(F)F | ||
| Standard InChIKey | RYYNGWLOYLRZLK-RBUKOAKNSA-N | ||
| Standard InChI | InChI=1S/C23H25F3N6O2/c1-12(33)27-11-20(34)32-18-7-8-19(32)16-9-14(5-6-15(16)18)30-22-28-10-17(23(24,25)26)21(31-22)29-13-3-2-4-13/h5-6,9-10,13,18-19H,2-4,7-8,11H2,1H3,(H,27,33)(H2,28,29,30,31)/t18-,19+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ATP-competitive inhibitor of Aurora kinases A and B (IC50 values are 0.8 and 5 nM for recombinant Aurora B and Aurora A, respectively). Inhibits phosphorylation of Aurora B, histone H3 and Aurora A in cultured MDA-MB-231 cells (IC50 values are approximately 20, 50 and 150 nM respectively). Shown to block cytokinesis; inhibits cellular proliferation in several human tumor cell lines, including HCT-116, HL-60, A549 and H125, and in human xenograft mouse models. Orally available. |

PF-03814735 Dilution Calculator

PF-03814735 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1076 mL | 10.5379 mL | 21.0757 mL | 42.1514 mL | 52.6893 mL |
| 5 mM | 0.4215 mL | 2.1076 mL | 4.2151 mL | 8.4303 mL | 10.5379 mL |
| 10 mM | 0.2108 mL | 1.0538 mL | 2.1076 mL | 4.2151 mL | 5.2689 mL |
| 50 mM | 0.0422 mL | 0.2108 mL | 0.4215 mL | 0.843 mL | 1.0538 mL |
| 100 mM | 0.0211 mL | 0.1054 mL | 0.2108 mL | 0.4215 mL | 0.5269 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PF-03814735 is a potent, orally bioavailable, reversible inhibitor of both Aurora1 and Aurora2 kinases with IC50 values of 0.8nM and 5nM, respectively [1].
PF-03814735 is an ATP competitive inhibitor of Aurora kinases. It also shows inhibition of other protein kinases at 100nM, such as Flt1, FAK, TrkA, Met, and FGFR1. The immunofluorescence imaging analysis shows PF-03814735 can inhibit the phosphorylation of Aurora1, Aurora 2 as well as histone H3 in MDA-MB-231 cells. This inhibition is rapid and reversible. The inhibition of phosphorylated histone H3 also occurs in athymic mice bearing HCT-116 xenografts. PF-03814735 induces the formation of polyploid cells and multinucleated cells due to the block in cytokinesis secondary. Moreover, PF-03814735 treatment results in a reduction of cell proliferation in vitro (such as HL-60, A549, and H125) and a inhibition of tumor growth in vivo (human xenograft mouse models, such as A2780 ovarian carcinoma and HCT-116) [1].
References:
[1] Jani JP, Arcari J, Bernardo V, Bhattacharya SK, Briere D, Cohen BD, Coleman K, Christensen JG, Emerson EO, Jakowski A, Hook K, Los G, Moyer JD, Pruimboom-Brees I, Pustilnik L, Rossi AM, Steyn SJ, Su C, Tsaparikos K, Wishka D, Yoon K, Jakubczak JL. PF-03814735, an orally bioavailable small molecule aurora kinase inhibitor for cancer therapy. Mol Cancer Ther. 2010 Apr;9(4):883-94.
- Nemoralisin
Catalog No.:BCN4488
CAS No.:942480-13-9
- VKGILS-NH2
Catalog No.:BCC3953
CAS No.:942413-05-0
- Cycloart-24-ene-1alpha,2alpha,3beta-triol
Catalog No.:BCN7983
CAS No.:942407-97-8
- Scutebarbatine I
Catalog No.:BCN1026
CAS No.:960302-84-5
- 20-Dehydroeupatoriopicrin semiacetal
Catalog No.:BCN7370
CAS No.:94234-24-9
- PI 828
Catalog No.:BCC7494
CAS No.:942289-87-4
- SCH772984
Catalog No.:BCC1935
CAS No.:942183-80-4
- VTP-27999
Catalog No.:BCC2048
CAS No.:942142-51-0
- Methyl 3-indolecarboxylate
Catalog No.:BCN4487
CAS No.:942-24-5
- 8beta-(4'-Hydroxytigloyloxy)costunolide
Catalog No.:BCN7885
CAS No.:94190-32-6
- S-Ruxolitinib (INCB018424)
Catalog No.:BCC2201
CAS No.:941685-37-6
- INCB032304
Catalog No.:BCC6455
CAS No.:941685-27-4
- Walsuronoid B
Catalog No.:BCN4489
CAS No.:942582-15-2
- 1,5,15-Tri-O-methylmorindol
Catalog No.:BCN4490
CAS No.:942609-65-6
- 5-Hydroxy-7,8,2',5'-tetramethoxyflavone 5-O-glucoside
Catalog No.:BCN1302
CAS No.:942626-75-7
- Dihydromicromelin B
Catalog No.:BCN4491
CAS No.:94285-06-0
- Acetyldihydromicromelin A
Catalog No.:BCN4492
CAS No.:94285-22-0
- GSK1070916
Catalog No.:BCC2183
CAS No.:942918-07-2
- CCT129202
Catalog No.:BCC2187
CAS No.:942947-93-5
- 12-Hydroxyganoderic acid D
Catalog No.:BCN8051
CAS No.:942950-96-1
- 3beta-(2-O-alpha-L-Rhamnopyranosyl-beta-D-xylopyranosyloxy)-23-hydroxyoleana-12-ene-28-oic acid 6-O-beta-D-glucopyranosyl-beta-D-glucopyranosyl ester
Catalog No.:BCC8948
CAS No.:942997-00-4
- DGAT-1 inhibitor 2
Catalog No.:BCC1530
CAS No.:942999-61-3
- H-Phe(4-NH2)-OH
Catalog No.:BCC3152
CAS No.:943-80-6
- GAP-134 Hydrochloride
Catalog No.:BCC1589
CAS No.:943133-81-1
PF-03814735, an orally bioavailable small molecule aurora kinase inhibitor for cancer therapy.[Pubmed:20354118]
Mol Cancer Ther. 2010 Apr;9(4):883-94.
The Aurora family of highly related serine/threonine kinases plays a key role in the regulation of mitosis. Aurora1 and Aurora2 play important but distinct roles in the G(2) and M phases of the cell cycle and are essential for proper chromosome segregation and cell division. Overexpression and amplification of Aurora2 have been reported in different tumor types, including breast, colon, pancreatic, ovarian, and gastric cancer. PF-03814735 is a novel, potent, orally bioavailable, reversible inhibitor of both Aurora1 and Aurora2 kinases that is currently in phase I clinical trials for the treatment of advanced solid tumors. In intact cells, the inhibitory activity of PF-03814735 on the Aurora1 and Aurora2 kinases reduces levels of phospho-Aurora1, phosphohistone H3, and phospho-Aurora2. PF-03814735 produces a block in cytokinesis, resulting in inhibition of cell proliferation and the formation of polyploid multinucleated cells. Although PF-03814735 produces significant inhibition of several other protein kinases, the predominant biochemical effects in cellular assays are consistent with inhibition of Aurora kinases. Once-daily oral administration of PF-03814735 to mice bearing human xenograft tumors produces a reduction in phosphohistone H3 in tumors at doses that are tolerable and that result in significant inhibition of tumor growth. The combination of PF-03814735 and docetaxel in xenograft mouse tumor models shows additive tumor growth inhibition. These results support the clinical evaluation of PF-03814735 in cancer patients. Mol Cancer Ther; 9(4); 883-94. (c)2010 AACR.
An integrated genomic approach to identify predictive biomarkers of response to the aurora kinase inhibitor PF-03814735.[Pubmed:22222631]
Mol Cancer Ther. 2012 Mar;11(3):710-9.
PF-03814735 is a novel, reversible inhibitor of Aurora kinases A and B that finished a phase I clinical trial for the treatment of advanced solid tumors. To find predictive biomarkers of drug sensitivity, we screened a diverse panel of 87 cancer cell lines for growth inhibition upon PF-03814735 treatment. Small cell lung cancer (SCLC) and, to a lesser extent, colon cancer lines were very sensitive to PF-03814735. The status of the Myc gene family and retinoblastoma pathway members significantly correlated with the efficacy of PF-03814735. Whereas RB1 inactivation, intact CDKN2A/p16, and normal CCND1/Cyclin D1 status are hallmarks of SCLC, activation or amplification of any of the three Myc genes (MYC, MYCL1, and MYCN) clearly differentiated cell line sensitivity within the SCLC panel. By contrast, we found that expression of Aurora A and B were weak predictors of response. We observed a decrease in histone H3 phosphorylation and polyploidization of sensitive lines, consistent with the phenotype of Aurora B inhibition. In vivo experiments with two SCLC xenograft models confirmed the sensitivity of Myc gene-driven models to PF-03814735 and a possible schedule dependence of MYC/c-Myc-driven tumors. Altogether our results suggest that SCLC and other malignancies driven by the Myc family genes may be suitable indications for treatment by Aurora B kinase inhibitors.
Phase I, open-label, multicentre, dose-escalation, pharmacokinetic and pharmacodynamic trial of the oral aurora kinase inhibitor PF-03814735 in advanced solid tumours.[Pubmed:21852114]
Eur J Cancer. 2011 Oct;47(15):2256-64.
This phase I study (ClinicalTrials.gov ID: NCT00424632) evaluated the safe dose, pharmacokinetics, and pharmacodynamics of the aurora kinase A and B inhibitor, PF-03814735. Patients with advanced solid tumours received oral, once-daily (QD) PF-03814735 on Schedule A: days 1-5 (5-100mg); or Schedule B: days 1-10 (40-60mg) of 21-day cycles. Fifty-seven patients were treated: 32 and 25 on Schedules A and B, respectively. Dose-limiting toxicities were: febrile neutropenia (Schedule A); and increased levels of aspartate amino transferase, left ventricular dysfunction, and prolonged low-grade neutropenia (Schedule B). Maximum tolerated doses were 80mg QD (Schedule A) and 50mg QD (Schedule B). Common treatment-related adverse events were mainly mild to moderate and included diarrhoea, fatigue, nausea, and vomiting. Nineteen patients achieved stable disease, which was prolonged in four cases. PF-03814735 was rapidly absorbed and demonstrated linear pharmacokinetics up to 100mg QD; mean terminal half-life ranged from 14.4 to 23.6h. Aurora B activity, assessed by histone H3 phosphorylation in mitotic cells, decreased in tumour tissue from 10/12 patients evaluated (range: -70% to -3%). (18)F-fluorodeoxyglucose positron emission tomography demonstrated metabolic responses in only 1/21 patients. PF-03814735 was generally well tolerated with manageable toxicities, and a recommended phase II dose could be established for both schedules. Aurora B activity was inhibited in tumour tissue, but clinical or metabolic antitumour activity was limited.
Aurora kinase inhibitors: progress towards the clinic.[Pubmed:22350019]
Invest New Drugs. 2012 Dec;30(6):2411-32.
The Aurora kinases (serine/threonine kinases) were discovered in 1995 during studies of mutant alleles associated with abnormal spindle pole formation in Drosophila melanogaster. They soon became the focus of much attention because of their importance in human biology and association with cancer. Aurora kinases are essential for cell division and are primarily active during mitosis. Following their identification as potential targets for cancer chemotherapy, many Aurora kinase inhibitors have been discovered, and are currently under development. The binding modes of Aurora kinase inhibitors to Aurora kinases share specific hydrogen bonds between the inhibitor core and the back bone of the kinase hinge region, while others parts of the molecules may point to different parts of the active site via noncovalent interactions. Currently there are about 30 Aurora kinase inhibitors in different stages of pre-clinical and clinical development. This review summarizes the characteristics and status of Aurora kinase inhibitors in preclinical, Phase I, and Phase II clinical studies, with particular emphasis on the mechanisms of action and resistance to these promising anticancer agents. We also discuss the validity of Aurora kinases as oncology targets, on/off-target toxicities, and other important aspects of overall clinical performance and future of Aurora kinase inhibitors.


