GNF 5Bcr-Abl inhibitor CAS# 778277-15-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 778277-15-9 | SDF | Download SDF |
| PubChem ID | 44129660 | Appearance | Powder |
| Formula | C20H17F3N4O3 | M.Wt | 418.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 49 mg/mL (117.12 mM) *"≥" means soluble, but saturation unknown. | ||
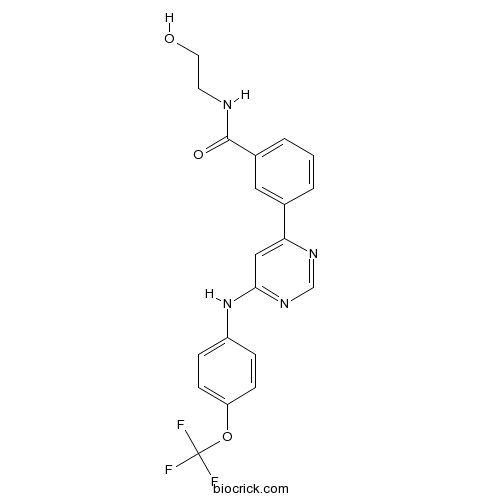
| Chemical Name | N-(2-hydroxyethyl)-3-[6-[4-(trifluoromethoxy)anilino]pyrimidin-4-yl]benzamide | ||
| SMILES | C1=CC(=CC(=C1)C(=O)NCCO)C2=CC(=NC=N2)NC3=CC=C(C=C3)OC(F)(F)F | ||
| Standard InChIKey | IIQUYGWWHIHOCF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H17F3N4O3/c21-20(22,23)30-16-6-4-15(5-7-16)27-18-11-17(25-12-26-18)13-2-1-3-14(10-13)19(29)24-8-9-28/h1-7,10-12,28H,8-9H2,(H,24,29)(H,25,26,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, non-ATP competitive allosteric inhibitor of Bcr-Abl (IC50 = 220 nM for wild-type Abl). Binds the myristate-binding site of Abl. Acts in combination with nilotinib to inhibit T315I Bcr-Abl in vitro and in vivo. Analog of GNF 2. |

GNF 5 Dilution Calculator

GNF 5 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3902 mL | 11.9511 mL | 23.9023 mL | 47.8046 mL | 59.7557 mL |
| 5 mM | 0.478 mL | 2.3902 mL | 4.7805 mL | 9.5609 mL | 11.9511 mL |
| 10 mM | 0.239 mL | 1.1951 mL | 2.3902 mL | 4.7805 mL | 5.9756 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.478 mL | 0.9561 mL | 1.1951 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.478 mL | 0.5976 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GNF-5 is an analogue of GNF-2 and a selective non-ATP competitive inhibitor of Bcr-Abl with an IC50 value of 0.1 to >10 µM in various cancer cell lines.
Bcr-Abl is a fusion gene that results from the head-to-tail fusion of the Bcr and Abl genes[1]. Bcr-Abl upregulates production of tyrosine kinase and plays a central role in the pathogenesis of chronic myelogenous leukemia (CML) [1].
GNF-5 has the same chemical structure as its parent molecule (GNF-2) with the exception of N-hydroxyethyl carboxamide at its 4-position and such modification provided GNF-5 a longer half-life from (2.30 hrs)[2]. Similar with GNF-2, GNF-5 allosterically inhibits the proliferation of Bcr-Abl positive cell by binding to the myristate-binding site of Abl and induces cell apoptosis[3]. In steady-state kinetic analyses, GNF-5 was able to inhibit wild type Abl with an IC50 value of 0.22 µM[2]. In addition, GNF-5 also has a similar effectiveness against various imatinib® resistance cell lines: In E255V and T315I mutant Ba/F3 cells, a 12-day incubation of GNF-5 2 was able to inhibit the proliferation of cells with a IC50 value of 0.38 and 5 µM, respectively[2].
In mice injected with wild-type Bcr-Abl and luciferase expressing Ba/F3 cells, continuous injection of GNF-5 for 7 days (50 mg/kg, twice per day) normalized peripheral blood cell counts, as well as spleen size[2]. When treating mice that injected with imatinib® resistance T315I Bcr–Abl-transduced bone marrow, daily injection of GNF-5 (75 mg/ kg, twice per day) significantly extended the survival day of mice from 24 days to 22 days[2].
References:
[1]. Rumpold, H. & Webersinke, G. 2011. Molecular pathogenesis of Philadelphia-positive chronic myeloid leukemia - is it all BCR-ABL? Curr Cancer Drug Targets, 11, 3-19.
[2]. Zhang, J., Adrian, F. J., Jahnke, W., et al. 2010. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature, 463, 501-506.
[3]. Karunakaran, U., Park, S. J., Jun, D. Y., et al. Non-receptor tyrosine kinase inhibitors enhances β-cell survival by suppressing the PKCδ signal transduction pathway in streptozotocin – induced β-cell apoptosis. Cellular Signalling.
- GNF 2
Catalog No.:BCC3891
CAS No.:778270-11-4
- Carasinol B
Catalog No.:BCN8226
CAS No.:777857-86-0
- Dehydrocrebanine
Catalog No.:BCN4328
CAS No.:77784-22-6
- Potassium phosphate monobasic
Catalog No.:BCC7583
CAS No.:7778-77-0
- 1,2-Dihydrotanshinquinone
Catalog No.:BCN2477
CAS No.:77769-21-2
- 1,7-Dihydroxy-3-methoxy-2-prenylxanthone
Catalog No.:BCN1354
CAS No.:77741-58-3
- (-)-Toddanol
Catalog No.:BCN3429
CAS No.:77715-99-2
- Arctigenin
Catalog No.:BCN6291
CAS No.:7770-78-7
- 6'''-Feruloylspinosin
Catalog No.:BCN2802
CAS No.:77690-92-7
- Enoximone
Catalog No.:BCC7155
CAS No.:77671-31-9
- ent-11,16-Epoxy-15-hydroxykauran-19-oic acid
Catalog No.:BCN1355
CAS No.:77658-46-9
- ent-9-Hydroxy-15-oxo-19-kauranoic acid
Catalog No.:BCN1356
CAS No.:77658-45-8
- Nelumol A
Catalog No.:BCN4749
CAS No.:77836-86-3
- (1R)-(+)-Alpha-Pinene
Catalog No.:BCC8275
CAS No.:7785-70-8
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- Isopulegol
Catalog No.:BCN4974
CAS No.:7786-67-6
- Beta-Lipotropin (1-10), porcine
Catalog No.:BCC1009
CAS No.:77875-68-4
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- Longikaurin E
Catalog No.:BCN4329
CAS No.:77949-42-9
- Z-Glycinol
Catalog No.:BCC3095
CAS No.:77987-49-6
- Secologanin dimethyl acetal
Catalog No.:BCN4581
CAS No.:77988-07-9
- Mulberrofuran C
Catalog No.:BCN4032
CAS No.:77996-04-4
- Isophorone
Catalog No.:BCN8329
CAS No.:78-59-1
Surface Charge-Transfer Doping of Graphene Nanoflakes Containing Double-Vacancy (5-8-5) and Stone-Wales (55-77) Defects through Molecular Adsorption.[Pubmed:27432283]
Chemphyschem. 2016 Oct 18;17(20):3289-3299.
The adsorption of six electron donor-acceptor (D/A) organic molecules on various sizes of graphene nanoflakes (GNFs) containing two common defects, double-vacancy (5-8-5) and Stone-Wales (55-77), are investigated by means of ab initio DFT [M06-2X(-D3)/cc-pVDZ]. Different D/A molecules adsorb on a defect graphene (DG) surface with binding energies (DeltaEb ) of about -12 to -28 kcal mol(-1) . The DeltaEb values for adsorption of molecules on the Stone-Wales GNF surface are higher than those on the double vacancy GNF surface. Moreover, binding energies increase by about 10 % with an increase in surface size. The nature of cooperative weak interactions is analyzed based on quantum theory of atoms in molecules, noncovalent interactions plot, and natural bond order analyses, and the dominant interaction is compared for different molecules. Electron density population analysis is used to explain the n- and p-type character of defect graphene nanoflakes (DGNFs) and also the change in electronic properties and reactivity parameters of DGNFs upon adsorption of different molecules and with increasing DGNF size. Results indicate that the HOMO-LUMO energy gap (Eg ) of DGNFs decreases upon adsorption of molecules. However, by increasing the size of DGNFs, the Eg and chemical hardness of all complexes decrease and the electrophilicity index increases. Furthermore, the values of the chemical potential of acceptor-DGNF complexes decrease with increasing size, whereas those of donor-DGNF complexes increase.
Gerontoxanthone B from Maclura cochinchinensis var. gerontogea exhibits anti-inflammatory potential as an aryl hydrocarbon receptor agonist.[Pubmed:28662965]
Bioorg Med Chem. 2017 Aug 15;25(16):4253-4258.
The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcriptional factor belonging to the basic helix-loop-helix-Per-Ahr/Arnt-Sim family. In this study, we evaluated the AhR agonistic activities of 12 xanthones isolated from the roots of M. cochinchinensis var. gerontogea using HepG2 cells transfected with pX4TK-Luc reporter plasmids. Gerontoxanthone B (GXB) showed the most potent activity at a concentration of 10muM, and the activity was inhibited by AhR antagonists such as GNF-351. GXB also increased cytochrome P450 1A1 mRNA and protein levels in HepG2 cells. Similar to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin, however, GXB suppressed the IL-1beta-induced mRNA level of SAA1, an acute-phase response gene that is up-regulated AhR-dependently but XRE-independently. Thus, GXB shows XRE-dependent transcriptional activity and XRE-independent activity involving AhR.
Aromaticity of graphene nanoflakes in a new way: fragment analysis by combination of the nucleus-independent chemical shifts and the anisotropy of current induced density.[Pubmed:28726151]
J Mol Model. 2017 Aug;23(8):231.
A graphene nanoflake (GNF) is a polycyclic aromatic hydrocarbon (PAH) with a huge two-dimensional pi-conjugated carbon material in which a central benzene ring is surrounded by identical benzene-type rings through infinite alternant method. In this paper, we explore the structure-aromaticity relationship of the GNFs and the GNFs with hollow sites (GNFHs) by combining the nucleus-independent chemical shifts (NICS) with the anisotropy of the current induced density (ACID). Firstly, the benzene is a typical aromatic molecule (NICS = -9.671 ppm), GNFs 1-6 is darned with benzene and the corresponding GNFHs 1'-6'. Secondly, the NICS values of GNFs 1-6 alternately vary: -1.214 (1) > -13.847 (2) < -2.662 (3) > -14.530 (4) < -3.932 (5) > -13.978 (6) ppm, the GNFs (2, 4, 6) with even fragments of annulene have larger aromaticity than that of GNFs (1, 3, 5) with odd fragments of annulene. Significantly, the NICS values of GNFs 1-6 can also be fragment analyzed by the NICS values and ACID of benzene and corresponding GNFHs 1'-6'. The NICS values for GNFs (2, 4, 6) can be roughly estimated by the NICS value of benzene minus the NICS value of the GNFHs (2', 4', 6'), respectively. The NICS values for GNFs (1, 3, 5) can be roughly estimated by the NICS value of the GNFHs (1', 3', 5') minus the NICS value of benzene, respectively. We hope that the present work can provide a simple and reliable method for the rational design of the GNF with aromaticity, which may be used to understand the origin of the graphene nanoflake aromatic properties.
Diet-Induced Obesity and Ghrelin Effects on Pituitary Gonadotrophs: Immunohistomorphometric Study in Male Rats.[Pubmed:26862530]
Cell J. 2016 Winter;17(4):711-9. Epub 2016 Jan 17.
OBJECTIVE: The close relationship between energy metabolism, nutritional state, and reproductive physiology suggests that nutritional and metabolic disorders can disrupt normal reproductive function and fertility. Considering the importance of leptin and ghrelin effects in regulation of the hypothalamic-pituitary-gonadal axis, the objective of this study was to investigate the influence of obesity and centrally applied ghrelin on immunohistochemical appearance and quantitative morphology of the pituitary follicle-stimulating hormone (FSH) and luteinizing hormone (LH) producing cells in adult male rats. MATERIALS AND METHODS: In this experimental study, animals were given two differ- ent diets: normal-fat (NF) and high-fat (HF), for 4 weeks, corresponding to normal and positive energy balance (n=2x14), respectively. Each group was subsequently divided into two subgroups (n=7) receiving intracerebroventricular (ICV) injections of either ghrelin [G, 1 microg/5 microL phosphate buffered saline (PBS)] or vehicle (5 microL PBS, control group) every 24 hours for five consecutive days. RESULTS: Morphometric analyses showed that in HF control group, the percentage of FSH cells per unit volume of total pituitary gland tissue (in mum(3)), i.e. volume density (Vvc), was increased (P<0.05) by 9.1% in comparison with the NF controls. After ICV treatment with ghrelin, volume (Vc) and volume density (Vvc) of FSH cells in ghrelin+NF (GNF) and ghrelin+HF (GHF) groups remained unchanged in comparison with NF and HF controls. Volume of LH cells in HF control group was increased by 17% (P<0.05), but their Vvc was decreased by 8.3% (P<0.05) in comparison with NF controls. In GNF group, the volume of LH cells increased by 7% (P<0.05), in comparison with the NF controls, but in GHF group, the same parameter remained unchanged when compared with HF controls. The central application of ghrelin de- creased the Vvc of LH cells only in GNF group by 38.9% (P<0.05) in comparison with the NF control animals. CONCLUSION: The present study has shown that obesity and repetitive ICV administra- tion of low doses of ghrelin, in NF and HF rats, modulated the immunohistomorphometric features of gonadotrophs, indicating the importance of obesity and ghrelin in regulation of the reproductive function.
Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs.[Pubmed:28438606]
Dev Biol. 2017 Jun 1;426(1):56-68.
Limb synovial joints are composed of distinct tissues, but it is unclear which progenitors produce those tissues and how articular cartilage acquires its functional postnatal organization characterized by chondrocyte columns, zone-specific cell volumes and anisotropic matrix. Using novel Gdf5(CreERT2) (Gdf5-CE), Prg4-CE and Dkk3-CE mice mated to R26-Confetti or single-color reporters, we found that knee joint progenitors produced small non-migratory progenies and distinct local tissues over prenatal and postnatal time. Stereological imaging and quantification indicated that the columns present in juvenile-adult tibial articular cartilage consisted of non-daughter, partially overlapping lineage cells, likely reflecting cell rearrangement and stacking. Zone-specific increases in cell volume were major drivers of tissue thickening, while cell proliferation or death played minor roles. Second harmonic generation with 2-photon microscopy showed that the collagen matrix went from being isotropic and scattered at young stages to being anisotropic and aligned along the cell stacks in adults. Progenitor tracing at prenatal or juvenile stages showed that joint injury provoked a massive and rapid increase in synovial Prg4+ and CD44+/P75+ cells some of which filling the injury site, while neighboring chondrocytes appeared unresponsive. Our data indicate that local cell populations produce distinct joint tissues and that articular cartilage growth and zonal organization are mainly brought about by cell volume expansion and topographical cell rearrangement. Synovial Prg4+ lineage progenitors are exquisitely responsive to acute injury and may represent pioneers in joint tissue repair.
Allosteric interactions between the myristate- and ATP-site of the Abl kinase.[Pubmed:21264348]
PLoS One. 2011 Jan 10;6(1):e15929.
Abl kinase inhibitors targeting the ATP binding pocket are currently employed as potent anti-leukemogenic agents but drug resistance has become a significant clinical limitation. Recently, a compound that binds to the myristate pocket of Abl (GNF-5) was shown to act cooperatively with nilotinib, an ATP-competitive inhibitor to target the recalcitrant "T315I" gatekeeper mutant of Bcr-Abl. To uncover an explanation for how drug binding at a distance from the kinase active site could lead to inhibition and how inhibitors could combine their effects, hydrogen exchange mass spectrometry (HX MS) was employed to monitor conformational effects in the presence of both dasatinib, a clinically approved ATP-site inhibitor, and GNF-5. While dasatinib binding to wild type Abl clearly influenced Abl conformation, no binding was detected between dasatinib and T315I. GNF-5, however, elicited the same conformational changes in both wild type and T315I, including changes to dynamics within the ATP site located approximately 25 A from the site of GNF-5 interaction. Simultaneous binding of dasatinib and GNF-5 to T315I caused conformational and/or dynamics changes in Abl such that effects of dasatinib on T315I were the same as when it bound to wild type Abl. These results provide strong biophysical evidence that allosteric interactions play a role in Abl kinase downregulation and that targeting sites outside the ATP binding site can provide an important pharmacological tool to overcome mutations that cause resistance to ATP-competitive inhibitors.
Expanding the diversity of allosteric bcr-abl inhibitors.[Pubmed:20828158]
J Med Chem. 2010 Oct 14;53(19):6934-46.
Inhibition of Bcr-Abl kinase activity by imatinib for the treatment of chronic myeloid leukemia (CML) currently serves as the paradigm for targeting dominant oncogenes with small molecules. We recently reported the discovery of GNF-2 (1) and GNF-5 (2) as selective non-ATP competitive inhibitors of cellular Bcr-Abl kinase activity that target the myristate binding site. Here, we used cell-based structure-activity relationships to guide the optimization and diversification of ligands that are capable of binding to the myristate binding site and rationalize the findings based upon an Abl-compound 1 cocrystal. We elucidate the structure-activity relationships required to obtain potent antiproliferative activity against Bcr-Abl transformed cells and report the discovery of new compounds (5g, 5h, 6a, 14d, and 21j-I) that display improved potency or pharmacological properties. This work demonstrates that a variety of structures can effectively target the Bcr-Abl myristate binding site and provides new leads for developing drugs that can target this binding site.
Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors.[Pubmed:20072125]
Nature. 2010 Jan 28;463(7280):501-6.
In an effort to find new pharmacological modalities to overcome resistance to ATP-binding-site inhibitors of Bcr-Abl, we recently reported the discovery of GNF-2, a selective allosteric Bcr-Abl inhibitor. Here, using solution NMR, X-ray crystallography, mutagenesis and hydrogen exchange mass spectrometry, we show that GNF-2 binds to the myristate-binding site of Abl, leading to changes in the structural dynamics of the ATP-binding site. GNF-5, an analogue of GNF-2 with improved pharmacokinetic properties, when used in combination with the ATP-competitive inhibitors imatinib or nilotinib, suppressed the emergence of resistance mutations in vitro, displayed additive inhibitory activity in biochemical and cellular assays against T315I mutant human Bcr-Abl and displayed in vivo efficacy against this recalcitrant mutant in a murine bone-marrow transplantation model. These results show that therapeutically relevant inhibition of Bcr-Abl activity can be achieved with inhibitors that bind to the myristate-binding site and that combining allosteric and ATP-competitive inhibitors can overcome resistance to either agent alone.


