EnoximonePDE3 inhibitor CAS# 77671-31-9 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- CCT137690
Catalog No.:BCC2188
CAS No.:1095382-05-0
- CYC116
Catalog No.:BCC2181
CAS No.:693228-63-6
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- TAK-901
Catalog No.:BCC2180
CAS No.:934541-31-8
- PF-03814735
Catalog No.:BCC2184
CAS No.:942487-16-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 77671-31-9 | SDF | Download SDF |
| PubChem ID | 53708 | Appearance | Powder |
| Formula | C12H12N2O2S | M.Wt | 248.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
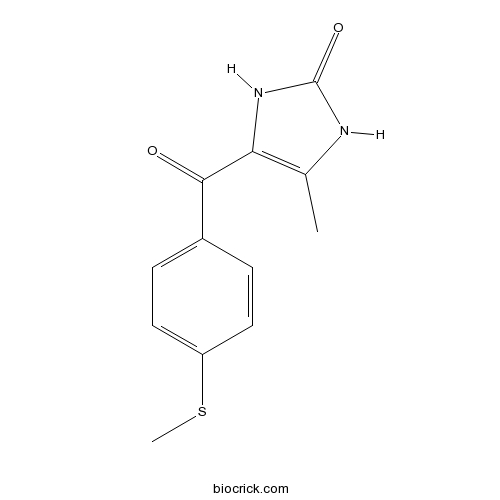
| Chemical Name | 4-methyl-5-(4-methylsulfanylbenzoyl)-1,3-dihydroimidazol-2-one | ||
| SMILES | CC1=C(NC(=O)N1)C(=O)C2=CC=C(C=C2)SC | ||
| Standard InChIKey | ZJKNESGOIKRXQY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H12N2O2S/c1-7-10(14-12(16)13-7)11(15)8-3-5-9(17-2)6-4-8/h3-6H,1-2H3,(H2,13,14,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of type III phosphodiesterase (PDE3). Increases intracellular cyclic AMP (cAMP) concentrations and enhances myocardial contractility. Also induces concentration-dependent vasodilation in vitro. |

Enoximone Dilution Calculator

Enoximone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0274 mL | 20.1369 mL | 40.2739 mL | 80.5477 mL | 100.6847 mL |
| 5 mM | 0.8055 mL | 4.0274 mL | 8.0548 mL | 16.1095 mL | 20.1369 mL |
| 10 mM | 0.4027 mL | 2.0137 mL | 4.0274 mL | 8.0548 mL | 10.0685 mL |
| 50 mM | 0.0805 mL | 0.4027 mL | 0.8055 mL | 1.611 mL | 2.0137 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4027 mL | 0.8055 mL | 1.0068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ent-11,16-Epoxy-15-hydroxykauran-19-oic acid
Catalog No.:BCN1355
CAS No.:77658-46-9
- ent-9-Hydroxy-15-oxo-19-kauranoic acid
Catalog No.:BCN1356
CAS No.:77658-45-8
- ent-9-Hydroxy-15-oxo-16-kauren-19-oic acid
Catalog No.:BCN1357
CAS No.:77658-39-0
- Pterokaurene L3
Catalog No.:BCN4583
CAS No.:77658-38-9
- Boc-D-Ala-OH
Catalog No.:BCC3049
CAS No.:7764-95-6
- Toddanone
Catalog No.:BCN3430
CAS No.:77636-08-9
- 1,4,5,6-Tetrahydroxy-7,8-diprenylxanthone
Catalog No.:BCN1358
CAS No.:776325-66-7
- Fmoc-HomoArg-OH
Catalog No.:BCC2645
CAS No.:776277-76-0
- Isoscopoletin
Catalog No.:BCN4582
CAS No.:776-86-3
- Nestoron
Catalog No.:BCC1797
CAS No.:7759-35-5
- Ingenol-3,4:5,20-diacetonide
Catalog No.:BCN2959
CAS No.:77573-44-5
- Ingenol-5,20-acetonide
Catalog No.:BCN2960
CAS No.:77573-43-4
- 6'''-Feruloylspinosin
Catalog No.:BCN2802
CAS No.:77690-92-7
- Arctigenin
Catalog No.:BCN6291
CAS No.:7770-78-7
- (-)-Toddanol
Catalog No.:BCN3429
CAS No.:77715-99-2
- 1,7-Dihydroxy-3-methoxy-2-prenylxanthone
Catalog No.:BCN1354
CAS No.:77741-58-3
- 1,2-Dihydrotanshinquinone
Catalog No.:BCN2477
CAS No.:77769-21-2
- Potassium phosphate monobasic
Catalog No.:BCC7583
CAS No.:7778-77-0
- Dehydrocrebanine
Catalog No.:BCN4328
CAS No.:77784-22-6
- Carasinol B
Catalog No.:BCN8226
CAS No.:777857-86-0
- GNF 2
Catalog No.:BCC3891
CAS No.:778270-11-4
- GNF 5
Catalog No.:BCC3892
CAS No.:778277-15-9
- Nelumol A
Catalog No.:BCN4749
CAS No.:77836-86-3
- (1R)-(+)-Alpha-Pinene
Catalog No.:BCC8275
CAS No.:7785-70-8
PDE3 inhibition with enoximone as first-line therapy for severe persistent pulmonary hypertension of the newborn during neonatal transport: a case report.[Pubmed:28096983]
Clin Case Rep. 2016 Nov 23;5(1):18-21.
Severe Persistent pulmonary hypertension of the newborn (PPHN) can be effectively treated with a PDE3 inhibitor as first-line treatment during neonatal transport when iNO is not readily available. Starting iNO as soon as possible is strongly advised because of the complementary actions of both therapeutics.
Use of enoximone in management of anaphylaxis complicated by labetalol use.[Pubmed:26504095]
BMJ Case Rep. 2015 Oct 26;2015. pii: bcr-2015-212432.
A 42-year-old woman with end-stage renal failure was admitted to the intensive care unit following resuscitation from a pulseless electrical activity cardiac arrest after intravenous piperacillin/tazobactam. Persistent bradycardia and hypotension, unresponsive to epinephrine and norepinephrine, were suspected to have been exacerbated by chronic labetalol therapy for resistant arterial hypertension. As an alternative, the non-adrenergic inotrope, Enoximone, was started. This, combined with thrombolysis for possible pulmonary embolism, heralded significant haemodynamic improvement, allowing weaning from inotropic support. A clear CT pulmonary angiogram 2 days post-arrest and significantly raised mast cell tryptase levels confirmed anaphylaxis rather than pulmonary embolism as the precipitating cause. We believe this to be the first case report of phosphodiesterase-III inhibitor use in the management of anaphylaxis complicated by alpha/beta-blockade, and discuss the mechanism behind this effect and comparison with the more commonly reported use of glucagon.
Conventional hemodynamic resuscitation may fail to optimize tissue perfusion: an observational study on the effects of dobutamine, enoximone, and norepinephrine in patients with acute myocardial infarction complicated by cardiogenic shock.[Pubmed:25084171]
PLoS One. 2014 Aug 1;9(8):e103978.
AIM: To investigate the effects of inotropic agents on parameters of tissue perfusion in patients with cardiogenic shock. METHODS AND RESULTS: Thirty patients with cardiogenic shock were included. Patients received dobutamine, Enoximone, or norepinephrine. We performed hemodynamic measurements at baseline and after titration of the inotropic agent until cardiac index (CI) >/= 2.5 L.min-1.m(-2) or mixed-venous oxygen saturation (SvO2) >/= 70% (dobutamine or Enoximone), and mean arterial pressure (MAP) >/= 70 mmHg (norepinephrine). As parameters of tissue perfusion, we measured central-peripheral temperature gradient (delta-T) and sublingual perfused capillary density (PCD). All patients reached predefined therapeutic targets. The inotropes did not significantly change delta-T. Dobutamine did not change PCD. Enoximone increased PCD (9.1 [8.9-10.2] vs. 11.4 [8.4-13.9] mm.mm(-2); p<0.05), and norepinephrine tended to decrease PCD (9.8 [8.5-11.9] vs. 8.8 [8.2-9.6] mm.mm-2, p = 0.08). Fifteen patients (50%) died within 30 days after admission. Patients who had low final PCD (10.3 mm.mm(-2); mortality 72% vs. 17%, p = 0.003). CONCLUSION: This study demonstrates the effects of commonly used inotropic agents on parameters of tissue perfusion in patients with cardiogenic shock. Despite hemodynamic optimization, tissue perfusion was not sufficiently restored in most patients. In these patients, mortality was high. Interventions directed at improving microcirculation may eventually help bridging the gap between improved hemodynamics and dismal patient outcome in cardiogenic shock.
Oral Enoximone as an Alternative to Protracted Intravenous Medication in Severe Pediatric Myocardial Failure.[Pubmed:27377525]
Pediatr Cardiol. 2016 Oct;37(7):1297-301.
Phosphodiesterase 3 inhibitors have been used successfully in pediatric patients with acute or chronic myocardial dysfunction over the last two decades. Their protracted continuous intravenous administration is associated with risk of infectious and thromboembolic complications. Weaning intravenous medication and starting oral angiotensin-converting enzyme (ACE) inhibitors and/or beta-blockers can be challenging. We reviewed retrospectively hospital records of 48 patients receiving oral Enoximone treatment in a single tertiary pediatric cardiac center between November 2005 and April 2014. Failure to wean from intravenous milrinone infusion and/or intolerance of ACE inhibitors and/or beta-blockers was indications for oral Enoximone treatment. Age of the patients ranged between 0.5 and 191 months (median 7.5 months) at the time of starting Enoximone treatment. There were 14 patients (29 %) with left ventricular dysfunction due to myocarditis or dilated cardiomyopathy and 34 patients (71 %) with myocardial dysfunction complicating congenital heart disease. Fifteen (44 %) of these 34 patients had left ventricular dysfunction, 13 (38 %) right ventricular dysfunction, and in 6 (18 %) both ventricles were failing. Duration of oral Enoximone treatment was between 3 days and 34 months (median of 2.3 months). Myocardial functional recovery allowed for weaning of Enoximone treatment in 15 patients (31 %) after 6 days-15 months (median 5 months). No adverse hemodynamic effects were noted. Blood stained gastric aspirates encountered in two patients resolved with concomitant milk administration. Based on our limited experience, oral Enoximone is a well-tolerated and promising alternative to intravenous medication and/or other commonly used oral medications in selected pediatric patients with chronic heart failure.
Pharmacological effects of ATI22-107 [2-(2-{2-[2-chloro-4-(6-oxo-1,4,5,6-tetrahydro-pyridazin-3-yl)-phenoxy]-acetylami no}-ethoxymethyl)-4-(2-chloro-phenyl)-6-methyl-1,4-dihydro-pyridine-3,5-dicarboxy lic acid dimethyl ester)], a novel dual pharmacophore, on myocyte calcium cycling and contractility.[Pubmed:15550574]
J Pharmacol Exp Ther. 2005 Feb;312(2):517-24.
Historically, inhibitors of type III phosphodiesterases (PDE-III) have been effective inotropes in mammalian myocardium, but their clinical utility has been limited by adverse events, including arrhythmias that are considered to be due to Ca(2+) overload. ATI22-107 [2-(2-{2-[2-chloro-4-(6-oxo-1,4,5,6-tetrahydro-pyridazin-3-yl)-phenoxy]-acetylami no}-ethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydro-pyridine-3,5-dicarboxyl ic acid dimethyl ester)], a novel, dual pharmacophore compound, was designed to simultaneously inhibit the cardiac phosphodiesterase (PDE-III) and produce inotropic effects, whereas inhibiting the L-type calcium channel (LTCC) was designed to minimize increases in diastolic Ca(2+). We compared the effects of ATI22-107 and Enoximone, a pure PDE-III inhibitor, on the Fluo-3 calcium transient in normal feline ventricular myocytes and trabeculae. Enoximone-induced dose-dependent increases in peak [Ca(2+)](i), diastolic [Ca(2+)](i), T50, and T75. ATI22-107 demonstrated similar dose-dependent increases in peak [Ca(2+)](i) at 300 nM and 1.0 microM doses, with no further increases at higher doses. Throughout the dosing range, ATI22-107 induced much smaller, if any, increases in diastolic [Ca(2+)](i), T(25), and T(75). Current measurement of LTCC via patch-clamp techniques revealed dose-dependent decreases in LTCC current with an increasing dose of ATI22-107, thereby validating the dual functionality of the drug that has been proposed in this study. Studies in isolated trabeculae demonstrated that Enoximone-induced a dose-dependent augmentation of the entire force-frequency relation in normal myocardium, whereas augmentation of contractility was only observed at lower stimulation frequencies with ATI22-107. These results demonstrate the effects of the LTCC-antagonizing moiety of ATI22-107 and suggest that the novel simultaneous combination of PDE-III and LTCC inhibition by one molecule may produce a favorable profile of limited inotropy without detrimental effects of increased diastolic [Ca(2+)](i).
Effect of phosphodiesterase inhibitors on human arteries in vitro.[Pubmed:8672353]
Br J Anaesth. 1996 Jan;76(1):122-9.
In the present study, we investigated if the relaxant effects of phosphodiesterase (PDE) III inhibitors on human vessels could be inhibited by a nitric oxide synthase blocker, L-NAME, or by a blocker of ATP-sensitive potassium channels (KATP), glibenclamide. The experiments were performed using an isometric myograph in isolated human s.c. small arteries obtained from healthy donors. After a priming procedure consisting of exposure to high potassium (120 mmol litre-1) solutions, phenylephrine 10 mumol litre-1 and an equilibrium period of 30 min, the preparations were contracted with a thromboxane A2 mimetic agent, U46619 1 mumol litre-1. Subsequently, cumulative concentration-response curves were constructed for the selective PDE III inhibitors amrinone, milrinone and Enoximone, and for theophylline and dipyridamole, with and without the addition of L-NAME 100 mumol litre-1 or glibenclamide 1 mumol litre-1. Addition of L-NAME to the organ bath resulted in significantly higher pEC50 values (-log of the concentration required for 50% relaxation) for milrinone compared with the control: 2.77 (SEM 0.24) mol litre-1 (n = 5) vs 3.49 (0.17) mol litre-1 (n = 7) (P < 0.05). There was no significant difference between any other group. From our data we conclude that the relaxant properties of amrinone, Enoximone, theophylline and dipyridamole are not dependent on nitric oxide release or on interaction with KATP channels. However, the effect of milrinone may be partly endothelium-dependent in human vessels in vitro.


