CCT137690Aurora A/B/C inhibitor CAS# 1095382-05-0 |
- SNS-314 Mesylate
Catalog No.:BCC2177
CAS No.:1146618-41-8
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- CYC116
Catalog No.:BCC2181
CAS No.:693228-63-6
- Barasertib (AZD1152-HQPA)
Catalog No.:BCC2168
CAS No.:722544-51-6
- MK-8745
Catalog No.:BCC3994
CAS No.:885325-71-3
- PF-03814735
Catalog No.:BCC2184
CAS No.:942487-16-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1095382-05-0 | SDF | Download SDF |
| PubChem ID | 25154041 | Appearance | Powder |
| Formula | C26H31BrN8O | M.Wt | 551.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 16.67 mg/mL (30.23 mM; Need ultrasonic) | ||
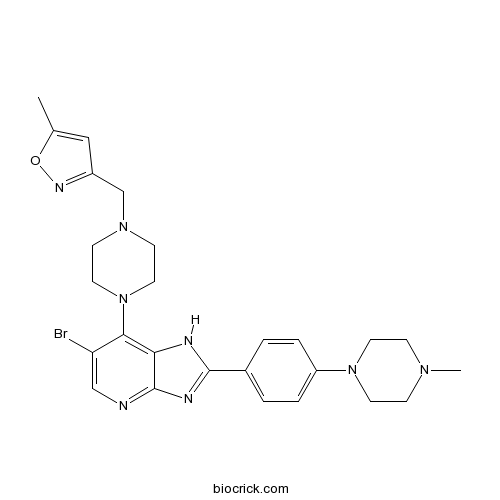
| Chemical Name | 3-[[4-[6-bromo-2-[4-(4-methylpiperazin-1-yl)phenyl]-1H-imidazo[4,5-b]pyridin-7-yl]piperazin-1-yl]methyl]-5-methyl-1,2-oxazole | ||
| SMILES | CC1=CC(=NO1)CN2CCN(CC2)C3=C4C(=NC=C3Br)N=C(N4)C5=CC=C(C=C5)N6CCN(CC6)C | ||
| Standard InChIKey | GFLQCBTXTRCREJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H31BrN8O/c1-18-15-20(31-36-18)17-33-9-13-35(14-10-33)24-22(27)16-28-26-23(24)29-25(30-26)19-3-5-21(6-4-19)34-11-7-32(2)8-12-34/h3-6,15-16H,7-14,17H2,1-2H3,(H,28,29,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of Aurora kinases (IC50 values are 0.015, 0.019 and 0.025 μM at Aurora A, Aurora C and Aurora B respectively). Displays antiproliferative activity in a range of human tumor cell lines. Orally bioavailable. |

CCT137690 Dilution Calculator

CCT137690 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8133 mL | 9.0665 mL | 18.133 mL | 36.266 mL | 45.3326 mL |
| 5 mM | 0.3627 mL | 1.8133 mL | 3.6266 mL | 7.2532 mL | 9.0665 mL |
| 10 mM | 0.1813 mL | 0.9067 mL | 1.8133 mL | 3.6266 mL | 4.5333 mL |
| 50 mM | 0.0363 mL | 0.1813 mL | 0.3627 mL | 0.7253 mL | 0.9067 mL |
| 100 mM | 0.0181 mL | 0.0907 mL | 0.1813 mL | 0.3627 mL | 0.4533 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CCT137690 is an orally bioavailable inhibitor of aurora kinases with IC50 values in a range from 15 to 25 nM [1].
Aurora kinase is a family of serine/threonine kinases including Aurora-A, B and C. They play important roles in the regulation of mitosis and take participate in the causation and progression of various tumors including ovarian, breast, glioma and colon. Therefore aurora kinases have been regarded as anti-cancer targets in cancer chemotherapeutics.CCT137690 is a selective small-molecular inhibitor of aurora kinases and showed anti-tumor activities both in vitro and in vivo. Besides that, CCT137690 exerted good stability in mouse liver microsomes [1].
When tested toward a panel of 94 kinases, CCT137690inhibited 80% activities of VEG-FR, Aurora-A, and FGF-R1 at concentration of 1 μM. It suppressed the phosphorylation of histone H3 caused by Aurora-B. The IC50 values of CCT137690 against Aurora-B and C were 25 and 19 nM, respectively. CCT137690 showed potent anti-proliferation effects on various kinds of tumors such as A2780 ovarian tumor cell (IC50 value of 140 nM), SW620 (IC50 value of 300 nM) and SW48 colon carcinoma (IC50 value of 157 nM). It caused cell cycle perturbations. In addition, CCT137690 was found to have synergistic effects with radiotherapy. It increased the sensitivity of SW620 cells to radiation. The combination treatment resulted in much more cell death through apoptosis [1 and 2].
In mice model bearing SW620 xenografts, administration of CCT137690slowed the growth of tumors without observed toxicity. The ratio of treat/control group based on tumor weight was 37% at the dose of 75 mg/kg. Besides that, CCT137690 was found to significantly reduced neuroblastoma tumor mass in MYCN transgenic mice, which meant CCT137690 could benefit patients with MYCN-amplified neuroblastoma [1 and 3].
References:
[1] Bavetsias V, Large J M, Sun C, et al. Imidazo [4, 5-b] pyridine derivatives as inhibitors of Aurora kinases: lead optimization studies toward the identification of an orally bioavailable preclinical development candidate. Journal of medicinal chemistry, 2010, 53(14): 5213-5228.
[2] Wu X, Liu W, Cao Q, et al. Inhibition of Aurora B by CCT137690 sensitizes colorectal cells to radiotherapy. J ExpClin Cancer Res, 2014, 33(1): 13.
[3] Faisal A, Vaughan L, Bavetsias V, et al. The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo. Molecular cancer therapeutics, 2011, 10(11): 2115-2123.
- ARQ 621
Catalog No.:BCC6534
CAS No.:1095253-39-6
- Rac1 Inhibitor W56
Catalog No.:BCC5886
CAS No.:1095179-01-3
- PF-04449913
Catalog No.:BCC5154
CAS No.:1095173-27-5
- JNJ-31020028
Catalog No.:BCC5516
CAS No.:1094873-14-9
- 3'-Methyl-4-O-methylhelichrysetin
Catalog No.:BCN4062
CAS No.:109471-13-8
- BIX 02189
Catalog No.:BCC2549
CAS No.:1094614-85-3
- BIX 02188
Catalog No.:BCC2550
CAS No.:1094614-84-2
- Fmoc-His(Trt)-OPfp
Catalog No.:BCC3502
CAS No.:109434-24-4
- Fmoc-Orn(Boc)-OH
Catalog No.:BCC3533
CAS No.:109425-55-0
- Fmoc-His(Trt)-OH
Catalog No.:BCC3501
CAS No.:109425-51-6
- SCH-1473759
Catalog No.:BCC1934
CAS No.:1094069-99-4
- CYM 5442 hydrochloride
Catalog No.:BCC7722
CAS No.:1094042-01-9
- RX 821002 hydrochloride
Catalog No.:BCC7021
CAS No.:109544-45-8
- Tacrolimus monohydrate
Catalog No.:BCC5284
CAS No.:109581-93-3
- Pinocembrin 7-acetate
Catalog No.:BCN5887
CAS No.:109592-60-1
- Topazolin
Catalog No.:BCN6833
CAS No.:109605-79-0
- Neocryptotanshinone
Catalog No.:BCN3158
CAS No.:109664-02-0
- SPK-601
Catalog No.:BCC1961
CAS No.:1096687-52-3
- MLN 2480
Catalog No.:BCC1771
CAS No.:1096708-71-2
- Murraol
Catalog No.:BCN5888
CAS No.:109741-38-0
- cis-Dehydroosthol
Catalog No.:BCN4735
CAS No.:109741-40-4
- 8 beta-(4-Acetoxy-5-hydroxytigloyloxy)costunolide
Catalog No.:BCN7123
CAS No.:109770-86-7
- Homopahutoxin
Catalog No.:BCN1812
CAS No.:109777-68-6
- 9R-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3079
CAS No.:1097882-33-1
Inhibition of Aurora B by CCT137690 sensitizes colorectal cells to radiotherapy.[Pubmed:24476310]
J Exp Clin Cancer Res. 2014 Jan 29;33:13.
Colorectal cancer is the third most commonly diagnosed cancer worldwide. Although surgery remains the best treatment for this disease, adjuvant chemotherapy and radiotherapy are also very important in clinical practice. However, the notorious refractory lack of responses to radiochemotherapy greatly limits the application of radiochemotherapy in the context of colorectal cancer.There is a growing interest in the role that Aurora B may play in colorectal cancer cell survival as well as other cancer subtypes. In the current study, we sought to ascertain whether blocking of Aurora B signaling machinery by a small molecule inhibitor, CCT137690, could synergize radiation-induced colorectal cancer cell death. Results showed that CCT137690 increases the sensitivity of SW620 cells to radiation. Mechanistic studies revealed that Aurora B-Survivin pathway may be involved in this synergistic effect.Taken together, our results for the first time show that Aurora B inhibition and radiation exert a synergistic effect, resulting in enhanced colorectal cancer cell death. This synergistic effect is clinically relevant as lower doses of radiation could be used for cancer treatment, and could provide significant clinical benefits in terms of colorectal cancer management, while reducing unwanted side-effects.
The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo.[Pubmed:21885865]
Mol Cancer Ther. 2011 Nov;10(11):2115-23.
Aurora kinases regulate key stages of mitosis including centrosome maturation, spindle assembly, chromosome segregation, and cytokinesis. Aurora A and B kinase overexpression has also been associated with various human cancers, and as such, they have been extensively studied as novel antimitotic drug targets. Here, we characterize the Aurora kinase inhibitor CCT137690, a highly selective, orally bioavailable imidazo[4,5-b]pyridine derivative that inhibits Aurora A and B kinases with low nanomolar IC(50) values in both biochemical and cellular assays and exhibits antiproliferative activity against a wide range of human solid tumor cell lines. CCT137690 efficiently inhibits histone H3 and transforming acidic coiled-coil 3 phosphorylation (Aurora B and Aurora A substrates, respectively) in HCT116 and HeLa cells. Continuous exposure of tumor cells to the inhibitor causes multipolar spindle formation, chromosome misalignment, polyploidy, and apoptosis. This is accompanied by p53/p21/BAX induction, thymidine kinase 1 downregulation, and PARP cleavage. Furthermore, CCT137690 treatment of MYCN-amplified neuroblastoma cell lines inhibits cell proliferation and decreases MYCN protein expression. Importantly, in a transgenic mouse model of neuroblastoma that overexpresses MYCN protein and is predisposed to spontaneous neuroblastoma formation, this compound significantly inhibits tumor growth. The potent preclinical activity of CCT137690 suggests that this inhibitor may benefit patients with MYCN-amplified neuroblastoma.
Imidazo[4,5-b]pyridine derivatives as inhibitors of Aurora kinases: lead optimization studies toward the identification of an orally bioavailable preclinical development candidate.[Pubmed:20565112]
J Med Chem. 2010 Jul 22;53(14):5213-28.
Lead optimization studies using 7 as the starting point led to a new class of imidazo[4,5-b]pyridine-based inhibitors of Aurora kinases that possessed the 1-benzylpiperazinyl motif at the 7-position, and displayed favorable in vitro properties. Cocrystallization of Aurora-A with 40c (CCT137444) provided a clear understanding into the interactions of this novel class of inhibitors with the Aurora kinases. Subsequent physicochemical property refinement by the incorporation of solubilizing groups led to the identification of 3-((4-(6-bromo-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl )piperazin-1-yl)methyl)-5-methylisoxazole (51, CCT137690) which is a potent inhibitor of Aurora kinases (Aurora-A IC(50) = 0.015 +/- 0.003 muM, Aurora-B IC(50) = 0.025 muM, Aurora-C IC(50) = 0.019 muM). Compound 51 is highly orally bioavailable, and in in vivo efficacy studies it inhibited the growth of SW620 colon carcinoma xenografts following oral administration with no observed toxicities as defined by body weight loss.


