Fmoc-His(Trt)-OHCAS# 109425-51-6 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 109425-51-6 | SDF | Download SDF |
| PubChem ID | 11422193 | Appearance | Powder |
| Formula | C40H33N3O4 | M.Wt | 619.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
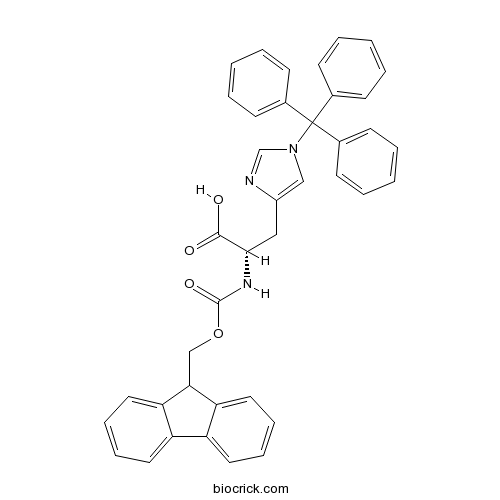
| Chemical Name | (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-(1-tritylimidazol-4-yl)propanoic acid | ||
| SMILES | C1=CC=C(C=C1)C(C2=CC=CC=C2)(C3=CC=CC=C3)N4C=C(N=C4)CC(C(=O)O)NC(=O)OCC5C6=CC=CC=C6C7=CC=CC=C57 | ||
| Standard InChIKey | XXMYDXUIZKNHDT-QNGWXLTQSA-N | ||
| Standard InChI | InChI=1S/C40H33N3O4/c44-38(45)37(42-39(46)47-26-36-34-22-12-10-20-32(34)33-21-11-13-23-35(33)36)24-31-25-43(27-41-31)40(28-14-4-1-5-15-28,29-16-6-2-7-17-29)30-18-8-3-9-19-30/h1-23,25,27,36-37H,24,26H2,(H,42,46)(H,44,45)/t37-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-His(Trt)-OH Dilution Calculator

Fmoc-His(Trt)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6137 mL | 8.0684 mL | 16.1368 mL | 32.2737 mL | 40.3421 mL |
| 5 mM | 0.3227 mL | 1.6137 mL | 3.2274 mL | 6.4547 mL | 8.0684 mL |
| 10 mM | 0.1614 mL | 0.8068 mL | 1.6137 mL | 3.2274 mL | 4.0342 mL |
| 50 mM | 0.0323 mL | 0.1614 mL | 0.3227 mL | 0.6455 mL | 0.8068 mL |
| 100 mM | 0.0161 mL | 0.0807 mL | 0.1614 mL | 0.3227 mL | 0.4034 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-His(Trt)-OH
- SCH-1473759
Catalog No.:BCC1934
CAS No.:1094069-99-4
- CYM 5442 hydrochloride
Catalog No.:BCC7722
CAS No.:1094042-01-9
- gamma-secretase modulator 2
Catalog No.:BCC1584
CAS No.:1093978-89-2
- VU 0155041
Catalog No.:BCC7615
CAS No.:1093757-42-6
- SRT2104 (GSK2245840)
Catalog No.:BCC1950
CAS No.:1093403-33-8
- Vibralactone B
Catalog No.:BCN6748
CAS No.:1093230-95-5
- LKB1 (AAK1 dual inhibitor)
Catalog No.:BCC1705
CAS No.:1093222-27-5
- 3-Hydroxy-4,15-dinor-1(5)-xanthen-12,8-olide
Catalog No.:BCN1625
CAS No.:1093207-99-8
- HNGF6A
Catalog No.:BCC8021
CAS No.:1093111-54-6
- B-Raf inhibitor 1
Catalog No.:BCC4182
CAS No.:1093100-40-3
- PLpro inhibitor
Catalog No.:BCC5302
CAS No.:1093070-14-4
- Angelol M
Catalog No.:BCN8271
CAS No.:1092952-64-1
- Fmoc-Orn(Boc)-OH
Catalog No.:BCC3533
CAS No.:109425-55-0
- Fmoc-His(Trt)-OPfp
Catalog No.:BCC3502
CAS No.:109434-24-4
- BIX 02188
Catalog No.:BCC2550
CAS No.:1094614-84-2
- BIX 02189
Catalog No.:BCC2549
CAS No.:1094614-85-3
- 3'-Methyl-4-O-methylhelichrysetin
Catalog No.:BCN4062
CAS No.:109471-13-8
- JNJ-31020028
Catalog No.:BCC5516
CAS No.:1094873-14-9
- PF-04449913
Catalog No.:BCC5154
CAS No.:1095173-27-5
- Rac1 Inhibitor W56
Catalog No.:BCC5886
CAS No.:1095179-01-3
- ARQ 621
Catalog No.:BCC6534
CAS No.:1095253-39-6
- CCT137690
Catalog No.:BCC2188
CAS No.:1095382-05-0
- RX 821002 hydrochloride
Catalog No.:BCC7021
CAS No.:109544-45-8
- Tacrolimus monohydrate
Catalog No.:BCC5284
CAS No.:109581-93-3
A 'conovenomic' analysis of the milked venom from the mollusk-hunting cone snail Conus textile--the pharmacological importance of post-translational modifications.[Pubmed:24055806]
Peptides. 2013 Nov;49:145-58.
Cone snail venoms provide a largely untapped source of novel peptide drug leads. To enhance the discovery phase, a detailed comparative proteomic analysis was undertaken on milked venom from the mollusk-hunting cone snail, Conus textile, from three different geographic locations (Hawai'i, American Samoa and Australia's Great Barrier Reef). A novel milked venom conopeptide rich in post-translational modifications was discovered, characterized and named alpha-conotoxin TxIC. We assign this conopeptide to the 4/7 alpha-conotoxin family based on the peptide's sequence homology and cDNA pre-propeptide alignment. Pharmacologically, alpha-conotoxin TxIC demonstrates minimal activity on human acetylcholine receptor models (100 muM, <5% inhibition), compared to its high paralytic potency in invertebrates, PD50 = 34.2 nMol kg(-1). The non-post-translationally modified form, [Pro](2,8)[Glu](16)alpha-conotoxin TxIC, demonstrates differential selectivity for the alpha3beta2 isoform of the nicotinic acetylcholine receptor with maximal inhibition of 96% and an observed IC50 of 5.4 +/- 0.5 muM. Interestingly its comparative PD50 (3.6 muMol kg(-1)) in invertebrates was ~100 fold more than that of the native peptide. Differentiating alpha-conotoxin TxIC from other alpha-conotoxins is the high degree of post-translational modification (44% of residues). This includes the incorporation of gamma-carboxyglutamic acid, two moieties of 4-trans hydroxyproline, two disulfide bond linkages, and C-terminal amidation. These findings expand upon the known chemical diversity of alpha-conotoxins and illustrate a potential driver of toxin phyla-selectivity within Conus.


