JNJ-31020028CAS# 1094873-14-9 |
- RG7388
Catalog No.:BCC1895
CAS No.:1229705-06-9
- MI-773
Catalog No.:BCC5155
CAS No.:1303607-07-9
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- Nutlin-3
Catalog No.:BCC2254
CAS No.:890090-75-2
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1094873-14-9 | SDF | Download SDF |
| PubChem ID | 25134625 | Appearance | Powder |
| Formula | C34H36FN5O2 | M.Wt | 565.68 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 21.5 mg/mL (38.01 mM; Need ultrasonic and warming) | ||
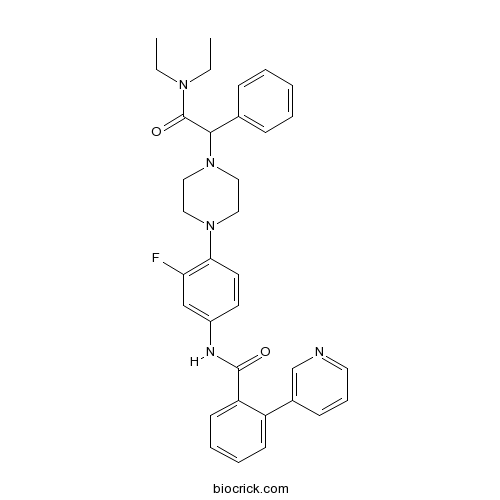
| Chemical Name | N-[4-[4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl]-3-fluorophenyl]-2-pyridin-3-ylbenzamide | ||
| SMILES | CCN(CC)C(=O)C(C1=CC=CC=C1)N2CCN(CC2)C3=C(C=C(C=C3)NC(=O)C4=CC=CC=C4C5=CN=CC=C5)F | ||
| Standard InChIKey | OVUNRYUVDVWTTE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C34H36FN5O2/c1-3-38(4-2)34(42)32(25-11-6-5-7-12-25)40-21-19-39(20-22-40)31-17-16-27(23-30(31)35)37-33(41)29-15-9-8-14-28(29)26-13-10-18-36-24-26/h5-18,23-24,32H,3-4,19-22H2,1-2H3,(H,37,41) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

JNJ-31020028 Dilution Calculator

JNJ-31020028 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7678 mL | 8.8389 mL | 17.6778 mL | 35.3557 mL | 44.1946 mL |
| 5 mM | 0.3536 mL | 1.7678 mL | 3.5356 mL | 7.0711 mL | 8.8389 mL |
| 10 mM | 0.1768 mL | 0.8839 mL | 1.7678 mL | 3.5356 mL | 4.4195 mL |
| 50 mM | 0.0354 mL | 0.1768 mL | 0.3536 mL | 0.7071 mL | 0.8839 mL |
| 100 mM | 0.0177 mL | 0.0884 mL | 0.1768 mL | 0.3536 mL | 0.4419 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
JNJ-31020028 is a selective brain penetrant antagonist of neuropeptide Y2 receptor with high affinity(pIC50=8.07, human; pIC50=8.22 rat); >100-fold selective versus human Y1/Y4/Y5 receptors. IC50 value: 8.07/8.22(human/rat pIC50) [1] Target: Y2 receptor antagonist in vitro: JNJ-31020028 was demonstrated to be an antagonist (pK(B) = 8.04 +/- 0.13) in functional assays [1]. in vivo: JNJ-31020028 occupied Y(2) receptor binding sites (approximately 90% at 10 mg/kg) after subcutaneous administration in rats [1]. Neither systemic (0, 15, 30, and 40 mg/kg, subcutaneously [s.c.]) nor intracerebroventricular (0.0, 0.3, and 1.0 nmol/rat) administration of JNJ-31020028 affected alcohol-reinforced lever pressing or relapse to alcohol seeking behavior following stress exposure. JNJ-31020028 (15 mg/kg, s.c.) did reverse the anxiogenic effects of withdrawal from a single bolus dose of alcohol on the elevated plus-maze, confirming the anxiolytic-like properties of NPY Y2 antagonism [2]. Chronic administration of JNJ-31020028 induced a decrease in immobility time in the forced swim test in OBX while had no effect in control animals [3].
References:
[1]. Shoblock JR, et al. In vitro and in vivo characterization of JNJ-31020028 (N-(4-{4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl}-3-fluorophenyl)-2-pyridin-3-ylbenzamide), a selective brain penetrant small molecule antagonist of the neuropeptide Y
[2]. Cippitelli A, et al. The novel, selective, brain-penetrant neuropeptide Y Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. Alcohol. 2011 Sep;45(6):567-76.
[3]. Morales-Medina JC, et al. Chronic administration of the Y2 receptor antagonist, JNJ-31020028, induced anti-depressant like-behaviors in olfactory bulbectomized rat. Neuropeptides. 2012 Dec;46(6):329-34.
- 3'-Methyl-4-O-methylhelichrysetin
Catalog No.:BCN4062
CAS No.:109471-13-8
- BIX 02189
Catalog No.:BCC2549
CAS No.:1094614-85-3
- BIX 02188
Catalog No.:BCC2550
CAS No.:1094614-84-2
- Fmoc-His(Trt)-OPfp
Catalog No.:BCC3502
CAS No.:109434-24-4
- Fmoc-Orn(Boc)-OH
Catalog No.:BCC3533
CAS No.:109425-55-0
- Fmoc-His(Trt)-OH
Catalog No.:BCC3501
CAS No.:109425-51-6
- SCH-1473759
Catalog No.:BCC1934
CAS No.:1094069-99-4
- CYM 5442 hydrochloride
Catalog No.:BCC7722
CAS No.:1094042-01-9
- gamma-secretase modulator 2
Catalog No.:BCC1584
CAS No.:1093978-89-2
- VU 0155041
Catalog No.:BCC7615
CAS No.:1093757-42-6
- SRT2104 (GSK2245840)
Catalog No.:BCC1950
CAS No.:1093403-33-8
- Vibralactone B
Catalog No.:BCN6748
CAS No.:1093230-95-5
- PF-04449913
Catalog No.:BCC5154
CAS No.:1095173-27-5
- Rac1 Inhibitor W56
Catalog No.:BCC5886
CAS No.:1095179-01-3
- ARQ 621
Catalog No.:BCC6534
CAS No.:1095253-39-6
- CCT137690
Catalog No.:BCC2188
CAS No.:1095382-05-0
- RX 821002 hydrochloride
Catalog No.:BCC7021
CAS No.:109544-45-8
- Tacrolimus monohydrate
Catalog No.:BCC5284
CAS No.:109581-93-3
- Pinocembrin 7-acetate
Catalog No.:BCN5887
CAS No.:109592-60-1
- Topazolin
Catalog No.:BCN6833
CAS No.:109605-79-0
- Neocryptotanshinone
Catalog No.:BCN3158
CAS No.:109664-02-0
- SPK-601
Catalog No.:BCC1961
CAS No.:1096687-52-3
- MLN 2480
Catalog No.:BCC1771
CAS No.:1096708-71-2
- Murraol
Catalog No.:BCN5888
CAS No.:109741-38-0
The discovery and synthesis of JNJ 31020028, a small molecule antagonist of the Neuropeptide Y Y(2) receptor.[Pubmed:21802951]
Bioorg Med Chem Lett. 2011 Sep 15;21(18):5552-6.
A series of small molecules based on a chemotype identified from our compound collection were synthesized and tested for binding affinity (IC(50)) at the human Neuropeptide Y Y(2) receptor (NPY Y(2)). Six of the 23 analogs tested possessed an NPY Y(2) IC(50)
Chronic administration of the Y2 receptor antagonist, JNJ-31020028, induced anti-depressant like-behaviors in olfactory bulbectomized rat.[Pubmed:23103057]
Neuropeptides. 2012 Dec;46(6):329-34.
Recent studies from our groups have shown that BIIE0246, a Y2 receptor antagonist, has antidepressant effect in olfactory bulbectomized (OBX) rat. However, its complex structure and high molecular weight limit its usefulness as an in vivo pharmacological tool. Alternatively, the novel and brain penetrant Y2 receptor antagonist, JNJ-31020028 is a useful tool to investigate the in vivo function of the Y2 receptor. In the present study, we evaluated the effect of chronic intracerebroventricular (icv) administration of JNJ-31020028 in a battery of behavioral tests in an animal model that mimics several deficits observed in the human depression, the OBX rat. Chronic administration of JNJ-31020028 induced a decrease in immobility time in the forced swim test in OBX while had no effect in control animals. Additionally, it decreased number of grooming events in OBX animals, but had no effects on some other behavioral deficits observed such as rearing and hyperlocomotion. Furthermore, JNJ-31020028 had no effect on behavior tests that are commonly used to evaluate anxiety, namely the social interaction test in both OBX and control animals. These data indicate that similar to BIIE0246, JNJ-31020028 also has antidepressant like effects in the OBX model.
Effects of a selective Y2R antagonist, JNJ-31020028, on nicotine abstinence-related social anxiety-like behavior, neuropeptide Y and corticotropin releasing factor mRNA levels in the novelty-seeking phenotype.[Pubmed:21497168]
Behav Brain Res. 2011 Sep 23;222(2):332-41.
An outbred rat model of novelty-seeking phenotype has predictive value for the expression of locomotor sensitization to nicotine. When experimentally naive rats are exposed to a novel environment, some display high rates of locomotor reactivity (HRs, scores ranking at top 1/3rd of the population), whereas some display low rates (LRs, scores ranking at bottom 1/3rd of the population). Basally, HRs display lower anxiety-like behavior compared to LRs along with higher neuropeptide Y (NPY) mRNA in the amygdala and the hippocampus. Following an intermittent behavioral sensitization to nicotine regimen and 1 wk of abstinence, HRs show increased social anxiety-like behavior in the social interaction test and robust expression of locomotor sensitization to a low dose nicotine challenge. These effects are accompanied by a deficit in NPY mRNA levels in the medial nucleus of the amygdala and the CA3 field of the hippocampus, and increases in Y2R mRNA levels in the CA3 field and corticotropin releasing factor (CRF) mRNA levels in the central nucleus of the amygdala. Systemic and daily injections of a Y2R antagonist, JNJ-31020028, during abstinence fully reverse nicotine-induced social anxiety-like behavior, the expression of locomotor sensitization to nicotine challenge, the deficit in the NPY mRNA levels in the amygdala and the hippocampus, as well as result an increase in Y2R mRNA levels in the hippocampus and the CRF mRNA levels in the amygdala in HRs. These findings implicate central Y2R in neuropeptidergic regulation of social anxiety in a behavioral sensitization to nicotine regimen in the LRHR rats.
PET brain imaging of neuropeptide Y2 receptors using N-11C-methyl-JNJ-31020028 in pigs.[Pubmed:24614224]
J Nucl Med. 2014 Apr;55(4):635-9.
UNLABELLED: Neuropeptide Y2 (NPY2) receptors are implicated in diverse brain disorders, but no suitable PET radiotracers are currently available for studying NPY2 receptors in the living brain. We developed a novel positron-emitting radioligand based on the NPY2 receptor antagonist JNJ-31020028 (N-(4-(4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl)-3-fluorophenyl)-2- pyridin-3-ylbenzamide) and used the radiotracer for PET brain imaging in pigs. METHODS: In vitro receptor autoradiography studies were performed to establish the anatomic distribution of NPY2 receptors in the pig brain. In vivo, baseline 90-min PET recordings of N-(11)C-methyl-JNJ-31020028 were conducted in anesthetized Yorkshire x Landrace pigs, concurrent with arterial blood sampling. Postchallenge scans were conducted after injection of unlabeled JNJ-31020028 as a pharmacologic intervention. Cyclosporine A was used to enhance levels of the PET radiotracer in the brain. The PET images were manually coregistered to a MR imaging atlas of the pig brain. Maps of the N-(11)C-methyl-JNJ-31020028 volume of distribution in the brain were prepared, and regional binding potentials of NPY2 receptors toward the radioligand were calculated using the simplified reference tissue method. RESULTS: In autoradiography studies, N-(11)C-methyl-JNJ-31020028 receptor binding sites were observed primarily in the hippocampus and were inhibited by unlabeled JNJ-31020028. In PET studies, N-(11)C-methyl-JNJ-31020028 was metabolized slowly in the bloodstream, with 25% of the (11)C-labeled parent compound remaining 30 min after injection. PET imaging showed baseline binding potentials of 0.64 +/- 0.07 in the thalamus, 0.55 +/- 0.02 in the caudate, and 0.49 +/- 0.03 in the hippocampus. Graphical reference region analyses demonstrated that N-(11)C-methyl-JNJ-31020028 binding was reversible; infusion of unlabeled JNJ-31020028 markedly displaced the PET radioligand from binding sites in the hippocampus, thalamus, caudate nucleus, and cerebellum but not in the corpus callosum, which served as reference region for nonspecific binding. CONCLUSION: N-(11)C-methyl-JNJ-31020028 has several suitable properties for PET neuroimaging of NPY2 receptors. First, it is metabolized slowly in the bloodstream of pigs. Second, using cyclosporine, the target-to-background ratio of N-(11)C-methyl-JNJ-31020028 is sufficient for estimating pharmacokinetic parameters. Third, N-(11)C-methyl-JNJ-31020028 binds reversibly and competitively to cerebral sites known to contain relatively high numbers of NPY2 receptors, such as the hippocampus, thalamus, caudate nucleus, and cerebellum. Fourth, white matter such as corpus callosum, known to contain negligible numbers of NPY2 receptors, can serve as a reference region for estimating binding potentials in brain regions. To our knowledge, there is no other radioligand with these favorable properties and with this specificity for NPY2 receptors, which makes N-(11)C-methyl-JNJ-31020028 the first candidate radioligand for PET investigations of NPY2 receptors in the living brain.


