Nutlin-3MDM2 antagonist,inhibits MDM2-p53 interaction CAS# 890090-75-2 |
- AMG232
Catalog No.:BCC3992
CAS No.:1352066-68-2
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- RITA (NSC 652287)
Catalog No.:BCC2238
CAS No.:213261-59-7
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- NSC 66811
Catalog No.:BCC2255
CAS No.:6964-62-1
- JNJ-26854165 (Serdemetan)
Catalog No.:BCC2240
CAS No.:881202-45-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 890090-75-2 | SDF | Download SDF |
| PubChem ID | 216345 | Appearance | Powder |
| Formula | C30H30Cl2N4O4 | M.Wt | 581.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
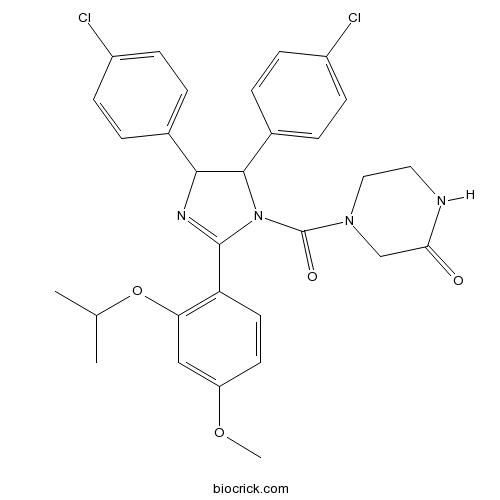
| Chemical Name | 4-[4,5-bis(4-chlorophenyl)-2-(4-methoxy-2-propan-2-yloxyphenyl)-4,5-dihydroimidazole-1-carbonyl]piperazin-2-one | ||
| SMILES | CC(C)OC1=C(C=CC(=C1)OC)C2=NC(C(N2C(=O)N3CCNC(=O)C3)C4=CC=C(C=C4)Cl)C5=CC=C(C=C5)Cl | ||
| Standard InChIKey | BDUHCSBCVGXTJM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nutlin-3 is a potent and selective antagonist of Mdm2 (RING finger-dependent ubiquitin protein ligase for itself and p53) with IC50 of 90 nM. | |||||
| Targets | Mdm2 | |||||
| IC50 | 90 nM | |||||

Nutlin-3 Dilution Calculator

Nutlin-3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7197 mL | 8.5985 mL | 17.1969 mL | 34.3938 mL | 42.9923 mL |
| 5 mM | 0.3439 mL | 1.7197 mL | 3.4394 mL | 6.8788 mL | 8.5985 mL |
| 10 mM | 0.172 mL | 0.8598 mL | 1.7197 mL | 3.4394 mL | 4.2992 mL |
| 50 mM | 0.0344 mL | 0.172 mL | 0.3439 mL | 0.6879 mL | 0.8598 mL |
| 100 mM | 0.0172 mL | 0.086 mL | 0.172 mL | 0.3439 mL | 0.4299 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nutlin-3, a tetra-substituted imidazoline, is a potent and selective small-molecule antagonist of murine double minute 2 (MDM2), which occupies the binding site of p53 in MDM2 and consequently prevent MDM2 binding to p53 leading to the disruption of the autoregulator feedback loop and the fostering of the p53 tumor suppressor network. It also binds to murine double minute 4 (MDM4), which is another component of the p35 tumor surveillance pathway. Nutlin-3 is being investigated as an antitumor agent for its antiangiogenic activity in cells through inhibiting endothelial cell migration, inducing cell cycle arrest, and increasing apoptotic tendency in endothelial cells.
Reference
Bernd R. Binder. A novel application for murine double minute 2 antagonists: the p53 tumor suppressor network also controls angiogenesis. Circ Res. 2007; 100: 13-14
- LUF6000
Catalog No.:BCC1710
CAS No.:890087-21-5
- 2'-Deoxyinosine
Catalog No.:BCN8544
CAS No.:890-38-0
- 2,4-Dihydroxyacetophenone
Catalog No.:BCN4441
CAS No.:89-84-9
- Thymol
Catalog No.:BCN3794
CAS No.:89-83-8
- Pulegone
Catalog No.:BCN3856
CAS No.:89-82-7
- (+)-Menthone
Catalog No.:BCC9239
CAS No.:89-80-5
- Neoisomenthol
Catalog No.:BCC8169
CAS No.:20752-34-5
- Mesalamine
Catalog No.:BCC4798
CAS No.:89-57-6
- Edaravone
Catalog No.:BCC2480
CAS No.:89-25-8
- Quinolinic acid
Catalog No.:BCC6573
CAS No.:89-00-9
- Dipsanoside B
Catalog No.:BCN2878
CAS No.:889678-64-2
- Dipsanoside A
Catalog No.:BCN2877
CAS No.:889678-62-0
- WDR5 0103
Catalog No.:BCC5626
CAS No.:890190-22-4
- Dregeoside A11
Catalog No.:BCN3993
CAS No.:89020-11-1
- erythro-Guaiacylglycerol beta-coniferyl ether
Catalog No.:BCN1315
CAS No.:890317-92-7
- VU 29
Catalog No.:BCC7936
CAS No.:890764-36-0
- VU 1545
Catalog No.:BCC7649
CAS No.:890764-63-3
- ML 349
Catalog No.:BCC5612
CAS No.:890819-86-0
- GSK 650394
Catalog No.:BCC4070
CAS No.:890842-28-1
- 24,25-Epoxytirucall-7-en-3,23-dione
Catalog No.:BCN4437
CAS No.:890928-81-1
- BAMB-4
Catalog No.:BCC5428
CAS No.:891025-25-5
- 4-O-Demethylisokadsurenin D
Catalog No.:BCN6650
CAS No.:89104-59-6
- Odoratisol A
Catalog No.:BCN7813
CAS No.:891182-93-7
- Betulin caffeate
Catalog No.:BCN4438
CAS No.:89130-86-9
Nutlin-3 reverses the epithelial-mesenchymal transition in gemcitabine-resistant hepatocellular carcinoma cells.[Pubmed:27430152]
Oncol Rep. 2016 Sep;36(3):1325-32.
Nutlin-3, a small molecule regulator of the tumor suppressor p53, targets the interaction between p53 and murine double minute 2 (MDM2) thereby promoting stabilization of p53 and subsequent p53dependent induction of apoptosis and cell cycle arrest. Recent studies have demonstrated that Nutlin3 plays a critical role in regulating tumor cell migration, invasion, metastasis, and drug resistance. Although these studies identified various biological functions of Nutlin3, our understanding of the exact molecular mechanisms of Nutlin3mediated antitumor activity remains incomplete. In this study, we elucidated a role of Nutlin3 in reversing the epithelialmesenchymal transition (EMT) in gemcitabine-resistant (GR) hepatocellular carcinoma (HCC) cells. We assessed the effect of Nutlin3 treatment on cell growth, migration, and invasion in both parental HCC cells and GR HCC cells. Moreover, we detected the expression of EMT markers in GR HCC cells treated with Nutlin3 by realtime RTPCR and western blot analysis, respectively. We found that Nutlin-3 inhibited cell migration and invasion in the GR HCC cells. Additionally, Nutlin3 treatment increased E-cadherin protein levels, but decreased the protein levels of vimentin, Snail and Slug in the GR HCC cells. Furthermore, we found that Smad2 was highly expressed in the GR HCC cells compared with their parental HCC cells, and Nutlin-3 treatment downregulated Smad2 expression in the GR HCC cells. Depletion of Smad2 retarded cell migration and regulated the expression of EMT markers in GR HCC cells similarly to Nutlin3 treatment. Our findings highlight an important role of Nutlin3 in reversing EMT in GR cells through regulation of Smad2 expression, suggesting that Nutlin-3 could be a potential agent for the treatment of HCC patients with gemcitabine resistance.
Nutlin-3 inhibits androgen receptor-driven c-FLIP expression, resulting in apoptosis of prostate cancer cells.[Pubmed:27729622]
Oncotarget. 2016 Nov 15;7(46):74724-74733.
Inhibition of androgen receptor (AR) signalling represents the conventional medical management of prostate cancer. Ultimately this treatment fails because tumors develop an incurable, castrate resistant phenotype, resulting in an unmet need for new treatments in prostate cancer. The AR remains a viable therapeutic target in castrate resistant disease, such that novel ways of downregulating AR activities are attractive as potential treatments. Here we describe a mechanism by which the AR can be downregulated by the MDM2 antagonist Nutlin-3, resulting in loss of pro-survival c-FLIP gene expression and apoptosis. We additionally show that loss of c-FLIP sensitises prostate cancer cells to Nutlin-3. Finally, we demonstrate that the unrelated MDM2 antagonist Mi-63 also impinges upon AR signalling, supporting the concept of future treatment of prostate cancer with MDM2 antagonists.
Nutlin-3, an Antagonist of MDM2, Enhances the Radiosensitivity of Esophageal Squamous Cancer with Wild-Type p53.[Pubmed:28341911]
Pathol Oncol Res. 2018 Jan;24(1):75-81.
Murine double minute 2 (MDM2) negatively regulates the activity of the p53 protein and plays a vital role in cell cycle arrest, apoptosis, and senescence mediated by p53. Nutlin-3, an antagonist of MDM2, is frequently used in anti-cancer studies. In many human tumors, Nutlin-3 stabilizes p53 status and enhances p53 expression in cells with wild-type p53. However, the effect of Nutlin-3 combined with radiotherapy on esophageal squamous cancer (ESCC) has not been reported. In this study, we examined whether Nutlin-3 increases the radiosensitivity of ESCC in vitro and in vivo.We chose two cell lines, ECA-109 (wild-type p53) and TE-13 (p53 mutated), for the following experiments. Cell proliferation and clonogenic survival experiments showed that Nutlin-3 inhibits the cell growth and colony formation of ECA-109 cells in a dose-dependent manner. Flow cytometry analysis showed that the apoptosis rate of ECA-109 cells co-treated with Nutlin-3 and irradiation(IR) was significantly increased compared with cells treated with irradiation or Nutlin-3 alone. Western blotting detected the expression of apoptosis-associated proteins in ECA-109 cells in response to Nutlin-3 and irradiation. These effects were not evident in TE-13 cells. Xenograft mouse models indicated that Nutlin-3 suppresses tumor growth and promotes radiosensitivity in the ESCC cell line ECA-109 in vivo. We have demonstrated that co-treatment of Nutlin-3 with irradiation can significantly inhibit the growth and improve the radiosensitivity of ESCC cells with wild-type p53. The study suggests that Nutlin-3 may be a potent therapeutic agent in conjunction with radiotherapy in ESCC.
Pre-clinical efficacy and synergistic potential of the MDM2-p53 antagonists, Nutlin-3 and RG7388, as single agents and in combined treatment with cisplatin in ovarian cancer.[Pubmed:27223080]
Oncotarget. 2016 Jun 28;7(26):40115-40134.
Ovarian cancer is the fifth leading cause of cancer-related female deaths. Due to serious side effects, relapse and resistance to standard chemotherapy, better and more targeted approaches are required. Mutation of the TP53 gene accounts for 50% of all human cancers. In the remaining malignancies, non-genotoxic activation of wild-type p53 by small molecule inhibition of the MDM2-p53 binding interaction is a promising therapeutic strategy. Proof of concept was established with the cis-imidazoline Nutlin-3, leading to the development of RG7388 and other compounds currently in early phase clinical trials. This preclinical study evaluated the effect of Nutlin-3 and RG7388 as single agents and in combination with cisplatin in a panel of ovarian cancer cell lines. Median-drug-effect analysis showed Nutlin-3 or RG7388 combination with cisplatin was additive to, or synergistic in a p53-dependent manner, resulting in increased p53 activation, cell cycle arrest and apoptosis, associated with increased p21WAF1 protein and/or caspase-3/7 activity compared to cisplatin alone. Although MDM2 inhibition activated the expression of p53-dependent DNA repair genes, the growth inhibitory and pro-apoptotic effects of p53 dominated the response. These data indicate that combination treatment with MDM2 inhibitors and cisplatin has synergistic potential for the treatment of ovarian cancer, dependent on cell genotype.


