PulegoneCAS# 89-82-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 89-82-7 | SDF | Download SDF |
| PubChem ID | 442495 | Appearance | Light brown liquid |
| Formula | C10H16O | M.Wt | 152.2 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Synonyms | (+)-p-Menth 4(8)-ene 3-one | ||
| Solubility | DMSO : ≥ 270 mg/mL (1773.63 mM) *"≥" means soluble, but saturation unknown. | ||
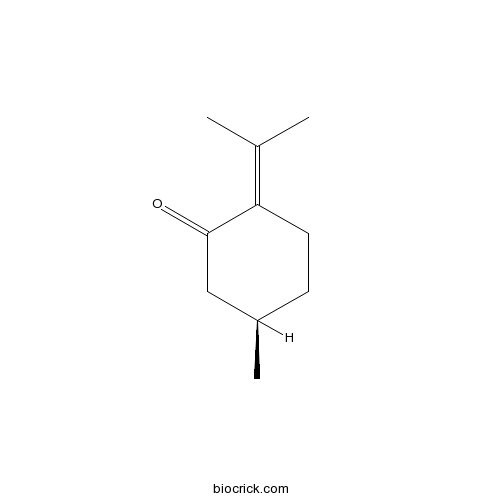
| Chemical Name | (5R)-5-methyl-2-propan-2-ylidenecyclohexan-1-one | ||
| SMILES | CC1CCC(=C(C)C)C(=O)C1 | ||
| Standard InChIKey | NZGWDASTMWDZIW-MRVPVSSYSA-N | ||
| Standard InChI | InChI=1S/C10H16O/c1-7(2)9-5-4-8(3)6-10(9)11/h8H,4-6H2,1-3H3/t8-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pulegone is a fragrance and flavour ingredient. Pulegone has cytotoxicity followed by regenerative cell proliferation is the MOA for Pulegone-induced urothelial tumors in female rats. Pulegone induces a verapamil-sensitive psychostimulant effect that appears to independ on the opening of L-type calcium channels. |
| Targets | GABA Receptor |
| In vivo | The aversive, anxiolytic-like, and verapamil-sensitive psychostimulant effects of pulegone.[Pubmed: 24790000]Biol Pharm Bull. 2014;37(5):771-8.
|
| Animal Research | Mode of action of pulegone on the urinary bladder of F344 rats.[Pubmed: 22499580]Toxicol Sci. 2012 Jul;128(1):1-8.Essential oils from mint plants, including peppermint and pennyroyal oils, are used at low levels as flavoring agents in various foods and beverages. Pulegone is a component of these oils. |

Pulegone Dilution Calculator

Pulegone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.5703 mL | 32.8515 mL | 65.703 mL | 131.406 mL | 164.2576 mL |
| 5 mM | 1.3141 mL | 6.5703 mL | 13.1406 mL | 26.2812 mL | 32.8515 mL |
| 10 mM | 0.657 mL | 3.2852 mL | 6.5703 mL | 13.1406 mL | 16.4258 mL |
| 50 mM | 0.1314 mL | 0.657 mL | 1.3141 mL | 2.6281 mL | 3.2852 mL |
| 100 mM | 0.0657 mL | 0.3285 mL | 0.657 mL | 1.3141 mL | 1.6426 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Menthone
Catalog No.:BCC9239
CAS No.:89-80-5
- Neoisomenthol
Catalog No.:BCC8169
CAS No.:20752-34-5
- Mesalamine
Catalog No.:BCC4798
CAS No.:89-57-6
- Edaravone
Catalog No.:BCC2480
CAS No.:89-25-8
- Quinolinic acid
Catalog No.:BCC6573
CAS No.:89-00-9
- Dipsanoside B
Catalog No.:BCN2878
CAS No.:889678-64-2
- Dipsanoside A
Catalog No.:BCN2877
CAS No.:889678-62-0
- Mogrol
Catalog No.:BCN8446
CAS No.:88930-15-8
- Mogroside IVe
Catalog No.:BCN3166
CAS No.:88915-64-4
- (-)-Xestospongin C
Catalog No.:BCC7002
CAS No.:88903-69-9
- Mogroside II-A2
Catalog No.:BCN3180
CAS No.:88901-45-5
- Mogroside II-A1
Catalog No.:BCN7926
CAS No.:88901-44-4
- Thymol
Catalog No.:BCN3794
CAS No.:89-83-8
- 2,4-Dihydroxyacetophenone
Catalog No.:BCN4441
CAS No.:89-84-9
- 2'-Deoxyinosine
Catalog No.:BCN8544
CAS No.:890-38-0
- LUF6000
Catalog No.:BCC1710
CAS No.:890087-21-5
- Nutlin-3
Catalog No.:BCC2254
CAS No.:890090-75-2
- WDR5 0103
Catalog No.:BCC5626
CAS No.:890190-22-4
- Dregeoside A11
Catalog No.:BCN3993
CAS No.:89020-11-1
- erythro-Guaiacylglycerol beta-coniferyl ether
Catalog No.:BCN1315
CAS No.:890317-92-7
- VU 29
Catalog No.:BCC7936
CAS No.:890764-36-0
- VU 1545
Catalog No.:BCC7649
CAS No.:890764-63-3
- ML 349
Catalog No.:BCC5612
CAS No.:890819-86-0
- GSK 650394
Catalog No.:BCC4070
CAS No.:890842-28-1
Mode of action of pulegone on the urinary bladder of F344 rats.[Pubmed:22499580]
Toxicol Sci. 2012 Jul;128(1):1-8.
Essential oils from mint plants, including peppermint and pennyroyal oils, are used at low levels as flavoring agents in various foods and beverages. Pulegone is a component of these oils. In a 2-year bioassay, oral administration of Pulegone slightly increased the urothelial tumor incidence in female rats. We hypothesized that its mode of action (MOA) involved urothelial cytotoxicity and increased cell proliferation, ultimately leading to tumors. Pulegone was administered by gavage at 0, 75, or 150 mg/kg body weight to female rats for 4 and 6 weeks. Fresh void urine and 18-h urine were collected for crystal and metabolite analyses. Urinary bladders were evaluated by light microscopy and scanning electron microscopy (SEM) and bromodeoxyuridine (BrdU) labeling index. Pulegone and its metabolites, piperitenone, piperitone, menthofuran, and menthone, were tested for cytotoxicity in rat (MYP3) and human (1T1) urothelial cells by the 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. No abnormal urinary crystals were observed by light microscopy. Urine samples (18-h) showed the presence of Pulegone, piperitone, piperitenone, and menthofuran in both treated groups. By SEM, bladders from treated rats showed superficial necrosis and exfoliation. There was a significant increase in the BrdU labeling index in the high-dose group. In vitro studies indicated that Pulegone and its metabolites, especially piperitenone, are excreted and concentrated in the urine at cytotoxic levels when Pulegone is administered at high doses to female rats. The present study supports the hypothesis that cytotoxicity followed by regenerative cell proliferation is the MOA for Pulegone-induced urothelial tumors in female rats.
The aversive, anxiolytic-like, and verapamil-sensitive psychostimulant effects of pulegone.[Pubmed:24790000]
Biol Pharm Bull. 2014;37(5):771-8.
We investigated the psychostimulant, rewarding, and anxiolytic-like effects of Pulegone. Possible interactions between Pulegone and menthol concerning their psychostimulant effect were also analyzed. General mouse activity after Pulegone treatment, and the interacitons between Pulegone and menthol, were determined in the open field. The anxiolytic-like activity, motor coordination and strength force were evaluated using the elevated plus maze (EPM), rotarod test and grasping test, respectively. The motivational properties of Pulegone were evaluated by pairing the drug effects on the mice with the least preferred compartment (previously determined) of a conditioned place preference (CPP) apparatus. Pulegone increased mouse locomotor activity and immobilization time. Verapamil, but not diltiazem, haloperidol or picrotoxin, decreased the psychostimulation induced by Pulegone. Pulegone also decreased grooming and rearing behaviors and caused motor incoordination and weakness at high doses. Pulegone increased the time spent by mice in the open arms of the EPM, and flumazenil pre-treatment did not alter this effect. Pulegone either produced no CPP or induced conditioned place aversion. The changes in mouse ambulatory activity caused by the association of Pulegone with menthol were either lower than those predicted by the theoretical curve or not different from the predicted values. Therefore, Pulegone induces a verapamil-sensitive psychostimulant effect that appears to independ on the opening of L-type calcium channels. Pulegone has negative reinforcing properties and seems to possess anxiolytic-like actions unrelated to the benzodiazepine site of the gamma-aminobutyric acid type A (GABAA) receptor. Finally, Pulegone might act in an addictive or synergic way with menthol.


