ThymolCAS# 89-83-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 89-83-8 | SDF | Download SDF |
| PubChem ID | 6989 | Appearance | Colorless crystals |
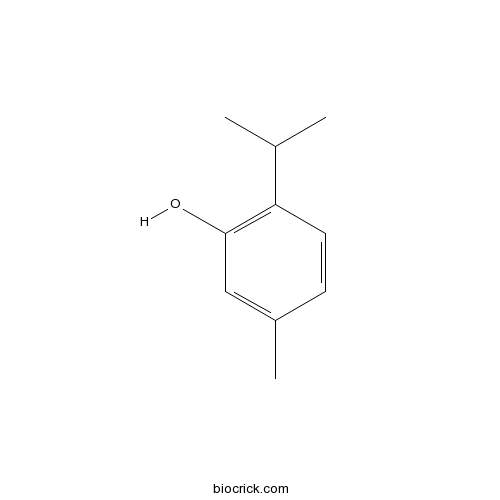
| Formula | C10H14O | M.Wt | 150.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (832.11 mM; Need ultrasonic) | ||
| Chemical Name | 5-methyl-2-propan-2-ylphenol | ||
| SMILES | CC1=CC(=C(C=C1)C(C)C)O | ||
| Standard InChIKey | MGSRCZKZVOBKFT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H14O/c1-7(2)9-5-4-8(3)6-10(9)11/h4-7,11H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Thymol is a positive allosteric modulator of human GABAA receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster, which has antioxidant, antimicrobial, anti-inflammatory, insecticidal and repellent properties. Thymol possesses anti-hepatotoxic activity, it prevents the CCl4-induced prolongation in pentobarbital sleeping time confirming hepatoprotectivity.Thymol has inhibitory effect on the release of human neutrophil elastase. |
| Targets | Antifection | GABA Receptor | Immunology & Inflammation related |
| In vitro | Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens.[Pubmed: 17334532]Braz. J. Med. Biol. Res. 2007, 40(3): 349-56.Dental caries and periodontal disease are associated with oral pathogens. Several plant derivatives have been evaluated with respect to their antimicrobial effects against such pathogenic microorganisms. Lippia sidoides Cham (Verbenaceae), popularly known as "Alecrim-pimenta" is a typical shrub commonly found in the Northeast of Brazil. Many plant species belonging to the genus Lippia yield very fragrant essential oils of potential economic value which are used by the industry for the commercial production of perfumes, creams, lotions, and deodorants. Since the leaves of L. sidoides are also extensively used in popular medicine for the treatment of skin wounds and cuts, the objective of the present study was to evaluate the composition and antimicrobial activity of L. sidoides essential oil. |
| Animal Research | Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) Sprague seeds against Anopheles stephensi.[Pubmed: 19343365 ]Parasitol. Res., 2009, 105(2):507-12.Essential oil of seeds of Trachyspermum ammi (Linn.) Sprauge and its pure constituent Thymol showed promising results when evaluated for larvicidal, oviposition-deterrent, vapor toxicity, and repellent activity against malarial vector, Anopheles stephensi. |

Thymol Dilution Calculator

Thymol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.6578 mL | 33.2889 mL | 66.5779 mL | 133.1558 mL | 166.4447 mL |
| 5 mM | 1.3316 mL | 6.6578 mL | 13.3156 mL | 26.6312 mL | 33.2889 mL |
| 10 mM | 0.6658 mL | 3.3289 mL | 6.6578 mL | 13.3156 mL | 16.6445 mL |
| 50 mM | 0.1332 mL | 0.6658 mL | 1.3316 mL | 2.6631 mL | 3.3289 mL |
| 100 mM | 0.0666 mL | 0.3329 mL | 0.6658 mL | 1.3316 mL | 1.6644 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pulegone
Catalog No.:BCN3856
CAS No.:89-82-7
- (+)-Menthone
Catalog No.:BCC9239
CAS No.:89-80-5
- Neoisomenthol
Catalog No.:BCC8169
CAS No.:20752-34-5
- Mesalamine
Catalog No.:BCC4798
CAS No.:89-57-6
- Edaravone
Catalog No.:BCC2480
CAS No.:89-25-8
- Quinolinic acid
Catalog No.:BCC6573
CAS No.:89-00-9
- Dipsanoside B
Catalog No.:BCN2878
CAS No.:889678-64-2
- Dipsanoside A
Catalog No.:BCN2877
CAS No.:889678-62-0
- Mogrol
Catalog No.:BCN8446
CAS No.:88930-15-8
- Mogroside IVe
Catalog No.:BCN3166
CAS No.:88915-64-4
- (-)-Xestospongin C
Catalog No.:BCC7002
CAS No.:88903-69-9
- Mogroside II-A2
Catalog No.:BCN3180
CAS No.:88901-45-5
- 2,4-Dihydroxyacetophenone
Catalog No.:BCN4441
CAS No.:89-84-9
- 2'-Deoxyinosine
Catalog No.:BCN8544
CAS No.:890-38-0
- LUF6000
Catalog No.:BCC1710
CAS No.:890087-21-5
- Nutlin-3
Catalog No.:BCC2254
CAS No.:890090-75-2
- WDR5 0103
Catalog No.:BCC5626
CAS No.:890190-22-4
- Dregeoside A11
Catalog No.:BCN3993
CAS No.:89020-11-1
- erythro-Guaiacylglycerol beta-coniferyl ether
Catalog No.:BCN1315
CAS No.:890317-92-7
- VU 29
Catalog No.:BCC7936
CAS No.:890764-36-0
- VU 1545
Catalog No.:BCC7649
CAS No.:890764-63-3
- ML 349
Catalog No.:BCC5612
CAS No.:890819-86-0
- GSK 650394
Catalog No.:BCC4070
CAS No.:890842-28-1
- 24,25-Epoxytirucall-7-en-3,23-dione
Catalog No.:BCN4437
CAS No.:890928-81-1
Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens.[Pubmed:17334532]
Braz J Med Biol Res. 2007 Mar;40(3):349-56.
Dental caries and periodontal disease are associated with oral pathogens. Several plant derivatives have been evaluated with respect to their antimicrobial effects against such pathogenic microorganisms. Lippia sidoides Cham (Verbenaceae), popularly known as "Alecrim-pimenta" is a typical shrub commonly found in the Northeast of Brazil. Many plant species belonging to the genus Lippia yield very fragrant essential oils of potential economic value which are used by the industry for the commercial production of perfumes, creams, lotions, and deodorants. Since the leaves of L. sidoides are also extensively used in popular medicine for the treatment of skin wounds and cuts, the objective of the present study was to evaluate the composition and antimicrobial activity of L. sidoides essential oil. The essential oil was obtained by hydro-distillation and analyzed by GC-MS. Twelve compounds were characterized, having as major constituents Thymol (56.7%) and carvacrol (16.7%). The antimicrobial activity of the oil and the major components was tested against cariogenic bacterial species of the genus Streptococcus as well as Candida albicans using the broth dilution and disk diffusion assays. The essential oil and its major components Thymol and carvacrol exhibited potent antimicrobial activity against the organisms tested with minimum inhibitory concentrations ranging from 0.625 to 10.0 mg/mL. The most sensitive microorganisms were C. albicans and Streptococcus mutans. The essential oil of L. sidoides and its major components exert promising antimicrobial effects against oral pathogens and suggest its likely usefulness to combat oral microbial growth.
Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) Sprague seeds against Anopheles stephensi.[Pubmed:19343365]
Parasitol Res. 2009 Aug;105(2):507-12.
Essential oil of seeds of Trachyspermum ammi (Linn.) Sprauge and its pure constituent Thymol showed promising results when evaluated for larvicidal, oviposition-deterrent, vapor toxicity, and repellent activity against malarial vector, Anopheles stephensi. Thymol was 1.6-fold more toxic than the oil toward fourth-instar larvae of A. stephensi with LD(50) values of 48.88 and 80.77 microg/ml, respectively. Egg laying by female adults of A. stephensi was much significantly reduced when exposed to vapors of Thymol compared to the oil of T. ammi seeds, and similar effects were recorded for subsequent egg hatching and larval survival. Vapor toxicity assay showed LC(50) value of 79.5 mg/mat for Thymol against adults of A. stephensi, whereas the crude oil exhibited the LC(50) value of 185.4 mg/mat. Thymol provided complete repellency toward A. stephensi adults at the dose of 25.0 mg/mat after 1 h duration, whereas same degree of repellency was obtained by the oil at the dose of 55.0 mg/mat, indicating its double-fold activity than the oil.


