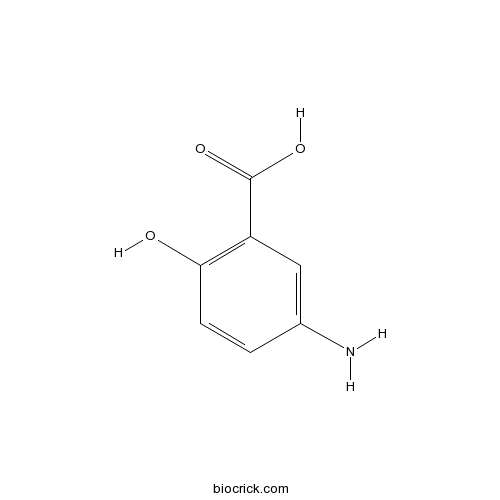
Mesalamine5-Aminosalicylic acid is an anti-inflammatory compound. CAS# 89-57-6 |
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Cefoselis Sulfate
Catalog No.:BCC4769
CAS No.:122841-12-7
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 89-57-6 | SDF | Download SDF |
| PubChem ID | 4075 | Appearance | Powder |
| Formula | C7H7NO3 | M.Wt | 153.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Mesalamine; 5-ASA; Mesalazine | ||
| Solubility | DMSO : ≥ 33.33 mg/mL (217.64 mM) H2O : 2 mg/mL (13.06 mM; ultrasonic and warming and heat to 60°C) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5-amino-2-hydroxybenzoic acid | ||
| SMILES | Nc1ccc(O)c(c1)C(O)=O | ||
| Standard InChIKey | KBOPZPXVLCULAV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H7NO3/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,9H,8H2,(H,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-Aminosalicylic acid acts as a specific PPARγ agonist and also inhibits p21-activated kinase 1 (PAK1) and NF-κB.In Vitro:5-Aminosalicylic acid (5-ASA) is a specific agonist for PPARγ, and only PPARγ but not PPARα or PPARδ induces p65 degradation. 5-Aminosalicylic acid induces degradation of p65 protein indicative of PPARγ's E3 ubiquitin ligase activity. 5-Aminosalicylic acid also inhibits PAK1 at the mRNA level which is suggestive of an additional mechanism independent of PPARγ ligand activation. 5-Aminosalicylic acid blocks NF-κB in intestinal epithelial cells (IECs) through inhibition of PAK1[1]. Pretreatment with 5-Aminosalicylic acid (5-ASA) or Nimesulide at different concentration (10-1000 μmol/L) for 12-96 h, inhibits the growth of HT-29 colon carcinoma cells in a dose and time-dependent manner. However, the suppression of 5-Aminosalicylic acid or Nimesulide has no statistical significance. The growth of HT-29 colon carcinoma cells is inhibited dose-dependently when pretreated with different doses of combined 5-Aminosalicylic acid and Nimesulide. Combined 5-Aminosalicylic acid (final concentration 100 μM) and Nimesulide (final concentration 10-1000 μM) inhibits the proliferation of HT-29 colon carcinoma cells in a dose-dependent manner, being more potent than corresponding dose of Nimesulide. Similarly, combined Nimesulide (final concentration 100 μM) and 5-Aminosalicylic acid (final concentration 10-1000 μM) also inhibits the proliferation of these cells dose-dependently, being more potent than corresponding dose of 5-Aminosalicylic acid[2].In Vivo:5-Aminosalicylic acid (5-ASA) has an antineoplastic effect in a xenograft tumor model. To evaluate the in vivo antineoplasic effect of 5-Aminosalicylic acid, SCID mice engrafted with HT-29 colon cancer cells are treated daily for 21 consecutive days with 5-Aminosalicylic acid at 50 mM. At the end of the treatment, a reduction of 80-86% of tumor weight and volume is observed in SCID mice receiving 5-Aminosalicylic acid compared with control mice or mice treated with GW9662 alone. The antineoplastic effect of 5-Aminosalicylic acid is already detectable after 10 days of 5-Aminosalicylic acid treatment. Similar results are obtained with mice treated with 5-Aminosalicylic acid at 5 mM. Antitumorigenic effect of 5-Aminosalicylic acid is completely abolished at 21 days by simultaneous intraperitoneal administration of GW9662. Thus, the observed antineoplastic effect of 5-Aminosalicylic acid is at least partially dependent on PPARγ[3]. References: | |||||

Mesalamine Dilution Calculator

Mesalamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.53 mL | 32.6499 mL | 65.2997 mL | 130.5995 mL | 163.2493 mL |
| 5 mM | 1.306 mL | 6.53 mL | 13.0599 mL | 26.1199 mL | 32.6499 mL |
| 10 mM | 0.653 mL | 3.265 mL | 6.53 mL | 13.0599 mL | 16.3249 mL |
| 50 mM | 0.1306 mL | 0.653 mL | 1.306 mL | 2.612 mL | 3.265 mL |
| 100 mM | 0.0653 mL | 0.3265 mL | 0.653 mL | 1.306 mL | 1.6325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
5-Aminosalicylic acid is an anti-inflammatory compound.
- Edaravone
Catalog No.:BCC2480
CAS No.:89-25-8
- Quinolinic acid
Catalog No.:BCC6573
CAS No.:89-00-9
- Dipsanoside B
Catalog No.:BCN2878
CAS No.:889678-64-2
- Dipsanoside A
Catalog No.:BCN2877
CAS No.:889678-62-0
- Mogrol
Catalog No.:BCN8446
CAS No.:88930-15-8
- Mogroside IVe
Catalog No.:BCN3166
CAS No.:88915-64-4
- (-)-Xestospongin C
Catalog No.:BCC7002
CAS No.:88903-69-9
- Mogroside II-A2
Catalog No.:BCN3180
CAS No.:88901-45-5
- Mogroside II-A1
Catalog No.:BCN7926
CAS No.:88901-44-4
- Mogroside III-A2
Catalog No.:BCN7925
CAS No.:88901-43-3
- Mogroside III-A1
Catalog No.:BCN3170
CAS No.:88901-42-2
- Mogroside IVa
Catalog No.:BCN3165
CAS No.:88901-41-1
- Neoisomenthol
Catalog No.:BCC8169
CAS No.:20752-34-5
- (+)-Menthone
Catalog No.:BCC9239
CAS No.:89-80-5
- Pulegone
Catalog No.:BCN3856
CAS No.:89-82-7
- Thymol
Catalog No.:BCN3794
CAS No.:89-83-8
- 2,4-Dihydroxyacetophenone
Catalog No.:BCN4441
CAS No.:89-84-9
- 2'-Deoxyinosine
Catalog No.:BCN8544
CAS No.:890-38-0
- LUF6000
Catalog No.:BCC1710
CAS No.:890087-21-5
- Nutlin-3
Catalog No.:BCC2254
CAS No.:890090-75-2
- WDR5 0103
Catalog No.:BCC5626
CAS No.:890190-22-4
- Dregeoside A11
Catalog No.:BCN3993
CAS No.:89020-11-1
- erythro-Guaiacylglycerol beta-coniferyl ether
Catalog No.:BCN1315
CAS No.:890317-92-7
- VU 29
Catalog No.:BCC7936
CAS No.:890764-36-0
First report of mesalamine (5-aminosalicylic acid) as the causative agent in a case of acute generalized exanthamous pustulosis.[Pubmed:28329471]
Dermatol Online J. 2017 Jan 15;23(1).
Acute generalized exanthamous pustulosis (AGEP)is a rare eruption of non-follicular sterile pustuleson a diffuse background of erythema and edema,commonly associated with fever and leukocytosis.Antibiotics are implicated in most cases; however,other drugs have been reported to cause AGEP. Wereport a case of a 73-year-old man with a historyof ulcerative colitis who presented with a diffusepustular rash, renal failure, elevated liver functiontests, and leukocytosis with neutrophilia. A week priorto admission, the patient was started on Mesalamineto treat colitis. Upon admission, a workup includinga skin biopsy was performed and was consistentwith AGEP. Mesalamine was discontinued, and thepatient's skin eruption, renal function, liver functiontests, and leukocytosis subsequently improved.Mesalamine has an unknown mechanism of action.However, it is thought to be an anti-inflammatoryagent that blocks the production of leukotrienesand prostaglandins and is an immunosuppressantthat increases the release of adenosine, whichinterferes with leukocyte function. The decrease inprostaglandin synthesis or deregulation of leukocytefunction caused by Mesalamine may be the etiologyin this case. Discontinuation of the offending agentleads to resolution of AGEP, as it did in this patient.
Mesalamine Enemas for Induction of Remission in Oral Mesalamine-refractory Pediatric Ulcerative Colitis: A Prospective Cohort Study.[Pubmed:28369299]
J Crohns Colitis. 2017 Aug 1;11(8):970-974.
Background: Paediatric ulcerative colitis [UC] is more extensive than adult disease, and more often refractory to Mesalamine. However, no prospective trials have evaluated Mesalamine enemas for inducing remission in children. Our goal was to evaluate the ability of Mesalamine enemas to induce remission in mild to moderate paediatric UC refractory to oral Mesalamine. Methods: This was an open-label arm of a previously reported randomised controlled trial of once-daily Mesalamine in active paediatric UC [MUPPIT trial]. Children aged 4-18 years, with a Paediatric Ulcerative Colitis Activity Index [PUCAI] score of 10-55, were enrolled after failing at least 3 weeks of full-dose oral Mesalamine. Patients treated with steroids or enemas in the previous month and those with isolated proctitis were excluded. Children received Pentasa(R) enemas 25 mg/kg [up to 1g] daily for 3 weeks with the previous oral dose. The primary endpoint was clinical remission by Week 3. Results: A total of 38 children were enrolled (mean age 14.6 +/- 2.3 years; 17/38 [45%] with extensive colitis). Clinical remission was obtained in 16 [42%] and response was obtained in 27 [71%] at Week 3. Eight children deteriorated and required steroids. There were no differences in baseline parameters between those who entered or failed to enter remission, including disease extent [43% in left-sided and 41% in extensive colitis] and disease activity [44% in mild and 41% in moderate activity]. Conclusion: Clinical remission can be markedly increased in children who are refractory to oral mesalamaine by adding Mesalamine enemas for 3 weeks, before commencing steroids.
2.4 g Mesalamine (Asacol 400 mg tablet) Once Daily is as Effective as Three Times Daily in Maintenance of Remission in Ulcerative Colitis: A Randomized, Noninferiority, Multi-center Trial.[Pubmed:28368909]
Inflamm Bowel Dis. 2017 May;23(5):822-832.
BACKGROUND: The noninferiority of pH-dependent release Mesalamine (Asacol) once daily (QD) to 3 times daily (TID) administration was investigated. METHODS: This was a phase 3, multicenter, randomized, double-blind, parallel-group, active-control study, with dynamic and stochastic allocation using central registration. Patients with ulcerative colitis in remission (a bloody stool score of 0, and an ulcerative colitis disease activity index of
Budesonide Multimatrix Is Efficacious for Mesalamine-refractory, Mild to Moderate Ulcerative Colitis: A Randomised, Placebo-controlled Trial.[Pubmed:28333362]
J Crohns Colitis. 2017 Jul 1;11(7):785-791.
Background and Aims: Safety and efficacy of budesonide multimatrix, an oral extended-release second-generation corticosteroid designed for targeted delivery throughout the colon, were examined for induction of remission in patients with mild to moderate ulcerative colitis refractory to baseline Mesalamine therapy. Methods: A randomised, double-blind, placebo-controlled, multicentre trial evaluated efficacy and safety of budesonide multimatrix for induction of remission [ulcerative colitis disease activity index score >/= 4 and Mesalamine >/= 2.4 g/day. Results: Combined clinical and endoscopic remission at Week 8 was achieved by 13.0% and 7.5% of patients receiving budesonide multimatrix [n = 230] or placebo [n = 228], respectively, in the modified intention-to-treat population [p = 0.049]. Clinical remission [ulcerative colitis disease activity index rectal bleeding and stool frequency subscale scores of 0] was similar in both groups [p = 0.70]. More patients receiving budesonide multimatrix vs placebo achieved endoscopic remission [ulcerative colitis disease activity index mucosal appearance subscale score of 0; 20.0% vs 12.3%; p = 0.02] and histological healing [27.0% vs 17.5%; p = 0.02]. Adverse event rates were similar [budesonide multimatrix, 31.8%; placebo, 27.1%]. Mean morning cortisol concentrations decreased at Weeks 2, 4, and 8 with budesonide multimatrix but remained within the normal range. Conclusion: Budesonide multimatrix was safe and efficacious for inducing clinical and endoscopic remission for mild to moderate ulcerative colitis refractory to oral Mesalamine therapy.


