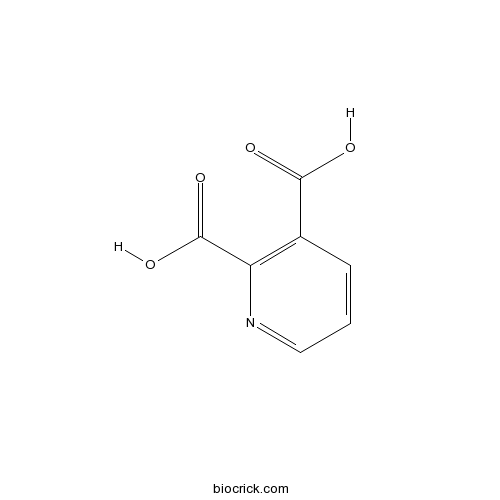
Quinolinic acidCAS# 89-00-9 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 89-00-9 | SDF | Download SDF |
| PubChem ID | 1066 | Appearance | Powder |
| Formula | C7H5NO4 | M.Wt | 167.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (199.44 mM; Need ultrasonic) | ||
| Chemical Name | pyridine-2,3-dicarboxylic acid | ||
| SMILES | C1=CC(=C(N=C1)C(=O)O)C(=O)O | ||
| Standard InChIKey | GJAWHXHKYYXBSV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-8-5(4)7(11)12/h1-3H,(H,9,10)(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous NMDA agonist and transmitter candidate. May distinguish between NMDA receptor subtypes. |

Quinolinic acid Dilution Calculator

Quinolinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.9837 mL | 29.9186 mL | 59.8372 mL | 119.6745 mL | 149.5931 mL |
| 5 mM | 1.1967 mL | 5.9837 mL | 11.9674 mL | 23.9349 mL | 29.9186 mL |
| 10 mM | 0.5984 mL | 2.9919 mL | 5.9837 mL | 11.9674 mL | 14.9593 mL |
| 50 mM | 0.1197 mL | 0.5984 mL | 1.1967 mL | 2.3935 mL | 2.9919 mL |
| 100 mM | 0.0598 mL | 0.2992 mL | 0.5984 mL | 1.1967 mL | 1.4959 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dipsanoside B
Catalog No.:BCN2878
CAS No.:889678-64-2
- Dipsanoside A
Catalog No.:BCN2877
CAS No.:889678-62-0
- Mogrol
Catalog No.:BCN8446
CAS No.:88930-15-8
- Mogroside IVe
Catalog No.:BCN3166
CAS No.:88915-64-4
- (-)-Xestospongin C
Catalog No.:BCC7002
CAS No.:88903-69-9
- Mogroside II-A2
Catalog No.:BCN3180
CAS No.:88901-45-5
- Mogroside II-A1
Catalog No.:BCN7926
CAS No.:88901-44-4
- Mogroside III-A2
Catalog No.:BCN7925
CAS No.:88901-43-3
- Mogroside III-A1
Catalog No.:BCN3170
CAS No.:88901-42-2
- Mogroside IVa
Catalog No.:BCN3165
CAS No.:88901-41-1
- Mogroside IIe
Catalog No.:BCN3168
CAS No.:88901-38-6
- Mogroside IIIe
Catalog No.:BCN7924
CAS No.:88901-37-5
- Edaravone
Catalog No.:BCC2480
CAS No.:89-25-8
- Mesalamine
Catalog No.:BCC4798
CAS No.:89-57-6
- Neoisomenthol
Catalog No.:BCC8169
CAS No.:20752-34-5
- (+)-Menthone
Catalog No.:BCC9239
CAS No.:89-80-5
- Pulegone
Catalog No.:BCN3856
CAS No.:89-82-7
- Thymol
Catalog No.:BCN3794
CAS No.:89-83-8
- 2,4-Dihydroxyacetophenone
Catalog No.:BCN4441
CAS No.:89-84-9
- 2'-Deoxyinosine
Catalog No.:BCN8544
CAS No.:890-38-0
- LUF6000
Catalog No.:BCC1710
CAS No.:890087-21-5
- Nutlin-3
Catalog No.:BCC2254
CAS No.:890090-75-2
- WDR5 0103
Catalog No.:BCC5626
CAS No.:890190-22-4
- Dregeoside A11
Catalog No.:BCN3993
CAS No.:89020-11-1
Microglia activation contributes to quinolinic acid-induced neuronal excitotoxicity through TNF-alpha.[Pubmed:28315174]
Apoptosis. 2017 May;22(5):696-709.
It has been reported that activation of NF-kappaB is involved in excitotoxicity; however, it is not fully understood how NF-kappaB contributes to excitotoxicity. The aim of this study is to investigate if NF-kappaB contributes to Quinolinic acid (QA)-mediated excitotoxicity through activation of microglia. In the cultured primary cortical neurons and microglia BV-2 cells, the effects of QA on cell survival, NF-kappaB expression and cytokines production were investigated. The effects of BV-2-conditioned medium (BCM) on primary cortical neurons were examined. The effects of pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF-kappaB, and minocycline (MC), an inhibitor of microglia activation, on QA-induced excitotoxicity were assessed. QA-induced NF-kappaB activation and TNF-alpha secretion, and the roles of TNF-alpha in excitotoxicity were studied. QA at the concentration below 1 mM had no apparent toxic effects on cultured primary neurons or BV-2 cells. However, addition of QA-primed BCM to primary neurons did aggravate QA-induced excitotoxicity. The exacerbation of QA-induced excitotoxicity by BCM was partially ameliorated by inhibiting NF-kappaB and microglia activation. QA induced activation of NF-kappaB and upregulation of TNF-alpha in BV-2 cells. Addition of recombinant TNF-alpha mimicked QA-induced excitotoxic effects on neurons, and neutralizing TNF-alpha with specific antibodies partially abolished exacerbation of QA-induced excitotoxicity by BCM. These studies suggested that QA activated microglia and upregulated TNF-alpha through NF-kappaB pathway in microglia. The microglia-mediated inflammatory pathway contributed, at least in part, to QA-induced excitotoxicity.
Retraction notice to "Curcumin restores Nrf2 levels and prevents quinolinic acid-induced neurotoxicity " [JNB 24 (2013) 14-24].[Pubmed:28320533]
J Nutr Biochem. 2017 Apr;42:203.
This article has been retracted: please see Elsevier Policy on Article Withdrawal (http://www.elsevier.com/locate/withdrawalpolicy). This article has been retracted at the request of the Editor-in-Chief as it contains inappropriately manipulated images in Figure 7B. As such this article represents a severe abuse of the scientific publishing system. The scientific community takes a very strong view on this matter, and apologies are offered to the readers of the journal that this problem was not detected during the submission process.
Beta-trace Protein as a new non-invasive immunological Marker for Quinolinic Acid-induced impaired Blood-Brain Barrier Integrity.[Pubmed:28276430]
Sci Rep. 2017 Mar 9;7:43642.
Quinolinic acid, a macrophage/microglia-derived excitotoxin fulfills a plethora of functions such as neurotoxin, gliotoxin, and proinflammatory mediator, and it alters the integrity and cohesion of the blood-brain barrier in several pathophysiological states. Beta-trace protein (BTP), a monomeric glycoprotein, is known to indicate cerebrospinal fluid leakage. Thus, the prior aim of this study was to investigate whether BTP might non-invasively indicate Quinolinic acid-induced impaired blood-brain barrier integrity. The research hypotheses were tested in three subsamples with different states of immune activation (patients with HCV-infection and interferon-alpha, patients with major depression, and healthy controls). BTP has also been described as a sensitive marker in detecting impaired renal function. Thus, the renal function has been considered. Our study results revealed highest Quinolinic acid and highest BTP- levels in the subsample of patients with HCV in comparison with the other subsamples with lower or no immune activation (Quinolinic acid: F = 21.027, p < 0.001 [ANOVA]; BTP: F = 6.792, p < 0.01 [ANOVA]). In addition, a two-step hierarchical linear regression model showed that significant predictors of BTP levels are Quinolinic acid, glomerular filtration rate and age. The neurotoxin Quinolinic acid may impair blood-brain barrier integrity. BTP might be a new non-invasive biomarker to indicate Quinolinic acid-induced impaired blood-brain barrier integrity.
Apocynin protects against neurological damage induced by quinolinic acid by an increase in glutathione synthesis and Nrf2 levels.[Pubmed:28323011]
Neuroscience. 2017 May 14;350:65-74.
Apocynin (APO) is a well-known NADPH oxidase (NOX) inhibitor. However, several studies have reported its ability to increase glutathione (GSH) levels. Due to GSH is a major non-enzymatic antioxidant in brain, the aim of this study was to evaluate, in the striatum of control and Quinolinic acid (QUIN) injected rats, the effect of APO administration on: (1) GSH levels, (2) activity of some enzymes involved in the GSH metabolism, and (3) nuclear factor erythroid-2-related factor 2 (Nrf2) mRNA levels. Animals received QUIN 240nmol in right striatum and APO (5mg/kg, i.p.), 30min before and 60min after intrastriatal injection. APO treatment prevented the QUIN-induced histological damage to the striatum. In control rats, APO treatment increased GSH and Nrf2 mRNA levels and the activities of gamma-glutamylcysteine ligase (gamma-GCL), glutathione-S-transferase (GST) and glutathione peroxidase (GPx). On the other hand, APO treatment prevented the QUIN-induced decrease in GSH and Nrf2 levels, and in gamma-GCL and GPx activities. These data indicate that APO is able to increase GSH levels and the activity of proteins involved in its metabolism, which could be associated with its ability to increase the Nrf2 mRNA levels.
The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits.[Pubmed:8740453]
Neurochem Int. 1996 Apr;28(4):445-52.
Quinolinic acid is an endogenous neurotoxin with NMDA receptor agonist properties. As such it may be the etiologic agent in many diseases. In this paper the NMDA receptor agonist properties of Quinolinic acid, as well as those of homoQuinolinic acid, a non endogenous analogue, were investigated in Xenopus oocytes injected with 12-day-old rat cortical mRNA or with recombinant NMDA receptors. In oocytes injected with cortical mRNA, Quinolinic acid was a weak NMDA receptor agonist: millimolar concentrations were necessary to induce responses that were smaller than maximal responses induced by NMDA; homoQuinolinic acid and NMDA had similar affinities but different efficacies: maximal responses induced by homoQuinolinic acid were larger than maximal responses induced by NMDA. Cortical mRNA, as verified by RT-PCR and restriction analysis, contains various NMDA subunits. In order to investigate if the low affinity or efficacy of Quinolinic acid could be explained by receptor composition, the pharmacological properties of the putative agonists were investigated in oocytes expressing binary combinations of recombinant NMDA receptors. Quinolinic acid did not activate receptors containing NR1 + NR2C but did activate receptors containing NR1 + NR2A and NR1 + NR2B even if only at millimolar concentrations; homoQuinolinic acid activated all subunit combinations but was less efficient than NMDA only in the NR1 + NR2C subunit combination. The relative efficacies of Quinolinic acid and homoQuinolinic acid were evaluated by comparing the maximal responses induced by these agonists with those induced by NMDA and glutamate in the same oocytes. The rank order of potency was Quinolinic acid < NMDA < homoQuinolinic acid < or = glutamate for the NR1 + NR2A and NR1 + NR2B combinations whereas for NR1 + NR2C it was Quinolinic acid << << homoQuinolinic acid < NMDA < or = glutamate. The use of Quinolinic acid and homoQuinolinic acid may thus help to identify endogenous receptors containing the NR2C subunit.
Quinolinate differentiates between forebrain and cerebellar NMDA receptors.[Pubmed:1676371]
Eur J Pharmacol. 1991 Feb 26;194(1):123-5.
The potency of N-methyl-D-aspartate (NMDA), ibotenate, L-glutamate and quinolinate for inhibiting [3H]L-glutamate binding to rat brain NMDA receptors was determined by quantitative autoradiography. In contrast to NMDA, ibotenate and L-glutamate, quinolinate more potently displaced binding in forebrain regions than in the cerebellum. Of all drug-region combinations, only quinolinate affinity in the cerebellum was best described by a two-affinity component model (Ki = 24 and 275 microM; 45% high affinity). The cerebellum appears to contain a unique quinolinate-insensitive NMDA receptor subtype.


