DexpramipexoleNeuroprotective agent CAS# 104632-28-2 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- L-Stepholidine
Catalog No.:BCN2599
CAS No.:16562-13-3
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104632-28-2 | SDF | Download SDF |
| PubChem ID | 59868 | Appearance | Powder |
| Formula | C10H17N3S | M.Wt | 211.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
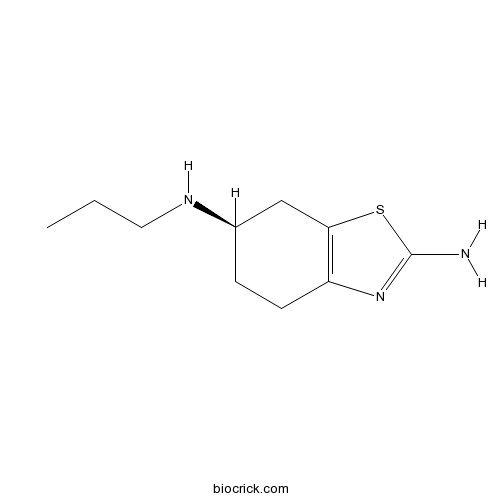
| Chemical Name | (6R)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine | ||
| SMILES | CCCNC1CCC2=C(C1)SC(=N2)N | ||
| Standard InChIKey | FASDKYOPVNHBLU-SSDOTTSWSA-N | ||
| Standard InChI | InChI=1S/C10H17N3S/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8/h7,12H,2-6H2,1H3,(H2,11,13)/t7-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dexpramipexole Dilution Calculator

Dexpramipexole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7319 mL | 23.6597 mL | 47.3194 mL | 94.6387 mL | 118.2984 mL |
| 5 mM | 0.9464 mL | 4.7319 mL | 9.4639 mL | 18.9277 mL | 23.6597 mL |
| 10 mM | 0.4732 mL | 2.366 mL | 4.7319 mL | 9.4639 mL | 11.8298 mL |
| 50 mM | 0.0946 mL | 0.4732 mL | 0.9464 mL | 1.8928 mL | 2.366 mL |
| 100 mM | 0.0473 mL | 0.2366 mL | 0.4732 mL | 0.9464 mL | 1.183 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dexpramipexole, also known as R-(+)-Pramipexole, is a neuroprotective agent and weak non-ergoline dopamine agonist. Dexpramipexole has been found to have neuroprotective effects and is being investigated for treatment of amyotrophic lateral sclerosis (ALS). Dexpramipexole reduces mitochondrial reactive oxygen species (ROS) production, inhibits the activation of apoptotic pathways, and increase cell survival in response to a variety of neurotoxins and β-amyloid neurotoxicity. Compared to the S-(-) isomer, Dexpramipexole has much lower dopamine agonist activity.
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Pramipexole
Catalog No.:BCC4467
CAS No.:104632-26-0
- Pramipexole dihydrochloride
Catalog No.:BCN2181
CAS No.:104632-25-9
- CGS 15943
Catalog No.:BCC7157
CAS No.:104615-18-1
- Caffeic acid phenethyl ester
Catalog No.:BCN2695
CAS No.:104594-70-9
- p-Menthan-3-one
Catalog No.:BCN3850
CAS No.:10458-14-7
- CAY10603
Catalog No.:BCC5542
CAS No.:1045792-66-2
- EC 23
Catalog No.:BCC6097
CAS No.:104561-41-3
- Bayogenin 3-O-beta-D-glucopyranoside
Catalog No.:BCN7868
CAS No.:104513-86-2
- Testosterone acetate
Catalog No.:BCC9165
CAS No.:1045-69-8
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- 3'-Methylflavokawin
Catalog No.:BCN3990
CAS No.:1044743-35-2
- H-Ile-NH2.HCl
Catalog No.:BCC2962
CAS No.:10466-56-5
- 7,3'-Di-O-methylorobol
Catalog No.:BCN6831
CAS No.:104668-88-4
- Iriflophenone 3-C-beta-D-glucopyranoside
Catalog No.:BCN1635
CAS No.:104669-02-5
- Lupiwighteone
Catalog No.:BCN4045
CAS No.:104691-86-3
- Ganoderic acid K
Catalog No.:BCN3039
CAS No.:104700-95-0
- Ganoderol B
Catalog No.:BCN5859
CAS No.:104700-96-1
- Ganoderol A
Catalog No.:BCN5860
CAS No.:104700-97-2
- Ganoderal A
Catalog No.:BCN2451
CAS No.:104700-98-3
- Boc-D-Glu(OtBu)-OH
Catalog No.:BCC3395
CAS No.:104719-63-3
- 8-Phenyloctanol
Catalog No.:BCC8791
CAS No.:10472-97-6
- 6-O-α-Maltosyl-β-cyclodextrin
Catalog No.:BCC8075
CAS No.:104723-60-6
- Mayteine
Catalog No.:BCN3098
CAS No.:104736-05-2
Dexpramipexole improves bioenergetics and outcome in experimental stroke.[Pubmed:28320070]
Br J Pharmacol. 2018 Jan;175(2):272-283.
BACKGROUND AND PURPOSE: Dexpramipexole, a drug recently tested in patients with amyotrophic lateral sclerosis (ALS,) is able to bind F1Fo ATP synthase and increase mitochondrial ATP production. Here, we have investigated its effects on experimental ischaemic brain injury. EXPERIMENTAL APPROACH: The effects of Dexpramipexole on bioenergetics, Ca(2+) fluxes, electrophysiological functions and death were evaluated in primary neural cultures and hippocampal slices exposed to oxygen-glucose deprivation (OGD). Effects on infarct volumes and neurological functions were also evaluated in mice following proximal or distal middle cerebral artery occlusion (MCAo). Distribution of Dexpramipexole within the ischaemic brain was evaluated by means of mass spectrometry imaging. KEY RESULTS: Dexpramipexole increased mitochondrial ATP production in cultured neurons or glia and reduces energy failure, prevents intracellular Ca(2+) overload and affords cytoprotection when cultures are exposed to OGD. This compound also counteracted ATP depletion, mitochondrial swelling, anoxic depolarization, loss of synaptic activity and neuronal death in hippocampal slices subjected to OGD. Post-ischaemic treatment with Dexpramipexole, at doses consistent with those already used in ALS patients, reduced brain infarct size and ameliorated neuroscore in mice subjected to transient or permanent MCAo. Notably, the concentrations of Dexpramipexole reached within the ischaemic penumbra equalled those found neuroprotective in vitro. CONCLUSION AND IMPLICATIONS: Dexpramipexole, a compound able to increase mitochondrial F1Fo ATP-synthase activity reduced ischaemic brain injury. These findings, together with the excellent brain penetration and favourable safety profile in humans, make Dexpramipexole a drug with realistic translational potential for the treatment of stroke. LINKED ARTICLES: This article is part of a themed section on Inventing New Therapies Without Reinventing the Wheel: The Power of Drug Repurposing. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.2/issuetoc.
The targeted eosinophil-lowering effects of dexpramipexole in clinical studies.[Pubmed:28178599]
Blood Cells Mol Dis. 2017 Mar;63:62-65.
Dexpramipexole, an orally bioavailable small molecule previously under clinical development in amyotrophic lateral sclerosis, was observed during routine safety hematology monitoring to demonstrate pronounced, dose- and time-dependent eosinophil-lowering effects, with minor reductions on other leukocyte counts. Analysis of hematology lab values across two double-blind, randomized placebo-controlled clinical trials at total daily doses ranging from 50mg to 300mg demonstrated that Dexpramipexole consistently and markedly lowered peripheral blood eosinophils. This effect developed after 1month on treatment, required 3-4months to reach its maximum, remained constant throughout treatment, and partially recovered to baseline levels upon drug withdrawal. All doses tested were well tolerated. The overall adverse event rate was similar for Dexpramipexole and placebo, and notably with no increase in infection-related adverse events associated with eosinophil-lowering effects. Given the reliance on and insufficiency of off-label chronic corticosteroid therapy for hypereosinophilic syndromes and other eosinophilic-associated diseases (EADs), a need exists for less toxic, more effective, targeted therapeutic alternatives. Further clinical studies are underway to assess the eosinophil-lowering effect of Dexpramipexole in the peripheral blood and target tissues of EAD patients and whether such reductions, if observed, produce clinically important benefits.
The mitochondrial complex V-associated large-conductance inner membrane current is regulated by cyclosporine and dexpramipexole.[Pubmed:25332381]
Mol Pharmacol. 2015 Jan;87(1):1-8.
Inefficiency of oxidative phosphorylation can result from futile leak conductance through the inner mitochondrial membrane. Stress or injury may exacerbate this leak conductance, putting cells, and particularly neurons, at risk of dysfunction and even death when energy demand exceeds cellular energy production. Using a novel method, we have recently described an ion conductance consistent with mitochondrial permeability transition pore (mPTP) within the c-subunit of the ATP synthase. Excitotoxicity, reactive oxygen species-producing stimuli, or elevated mitochondrial matrix calcium opens the channel, which is inhibited by cyclosporine A and ATP/ADP. Here we show that ATP and the neuroprotective drug Dexpramipexole (DEX) inhibited an ion conductance consistent with this c-subunit channel (mPTP) in brain-derived submitochondrial vesicles (SMVs) enriched for F1FO ATP synthase (complex V). Treatment of SMVs with urea denatured extramembrane components of complex V, eliminated DEX- but not ATP-mediated current inhibition, and reduced binding of [(14)C]DEX. Direct effects of DEX on the synthesis and hydrolysis of ATP by complex V suggest that interaction of the compound with its target results in functional conformational changes in the enzyme complex. [(14)C]DEX bound specifically to purified recombinant b and oligomycin sensitivity-conferring protein subunits of the mitochondrial F1FO ATP synthase. Previous data indicate that DEX increased the efficiency of energy production in cells, including neurons. Taken together, these studies suggest that modulation of a complex V-associated inner mitochondrial membrane current is metabolically important and may represent an avenue for the development of new therapeutics for neurodegenerative disorders.
Dexpramipexole is ineffective in two models of ALS related neurodegeneration.[Pubmed:25526593]
PLoS One. 2014 Dec 19;9(12):e91608.
Treatment options for people living with amyotrophic lateral sclerosis (ALS) are limited and ineffective. Recently, Dexpramipexole (RPPX) was advanced into human ALS clinical trials. In the current studies, we investigated RPPX in two parallel screening systems: 1) appropriately powered, sibling-matched, gender-balanced survival efficacy screening in high-copy B6-SJL-SOD1G93A/Gur1 mice, and 2) high-content neuronal survival screening in primary rat cortical neurons transfected with wild-type human TDP43 or mutant human TDP43. In both cases, we exposed the test systems to RPPX levels approximating those achieved in human Phase II clinical investigations. In SOD1G93A mice, no effect was observed on neuromotor disease progression or survival. In primary cortical neurons transfected with either mutant or wild-type human TDP43, a marginally significant improvement in a single indicator of neuronal survival was observed, and only at the 10 microM RPPX treatment. These systems reflect both mutant SOD1- and TDP43-mediated forms of neurodegeneration. The systems also reflect both complex non-cell autonomous and neuronal cell autonomous disease mechanisms. The results of these experiments, taken in context with results produced by other molecules tested in both screening systems, do not argue positively for further study of RPPX in ALS.


