Pramipexole dihydrochlorideDopamine receptor agonist CAS# 104632-25-9 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104632-25-9 | SDF | Download SDF |
| PubChem ID | 119569 | Appearance | Powder |
| Formula | C10H19Cl2N3S | M.Wt | 284.25 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (175.90 mM) *"≥" means soluble, but saturation unknown. | ||
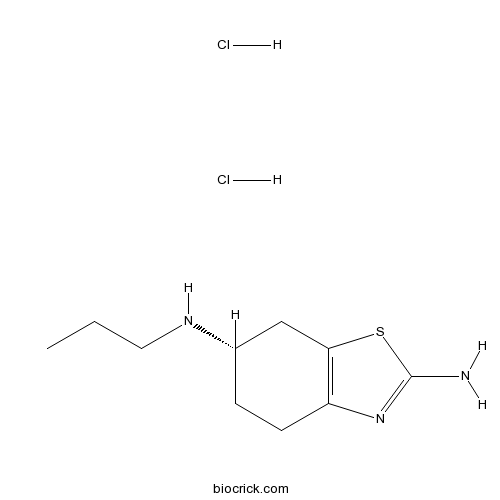
| Chemical Name | (6S)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine;dihydrochloride | ||
| SMILES | CCCNC1CCC2=C(C1)SC(=N2)N.Cl.Cl | ||
| Standard InChIKey | QMNWXHSYPXQFSK-KLXURFKVSA-N | ||
| Standard InChI | InChI=1S/C10H17N3S.2ClH/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8;;/h7,12H,2-6H2,1H3,(H2,11,13);2*1H/t7-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pramipexole dihydrochloride could be used to treat Parkinson disease. |
| Targets | Dopamine Receptor |

Pramipexole dihydrochloride Dilution Calculator

Pramipexole dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.518 mL | 17.5901 mL | 35.1803 mL | 70.3606 mL | 87.9507 mL |
| 5 mM | 0.7036 mL | 3.518 mL | 7.0361 mL | 14.0721 mL | 17.5901 mL |
| 10 mM | 0.3518 mL | 1.759 mL | 3.518 mL | 7.0361 mL | 8.7951 mL |
| 50 mM | 0.0704 mL | 0.3518 mL | 0.7036 mL | 1.4072 mL | 1.759 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3518 mL | 0.7036 mL | 0.8795 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pramipexole dihydrochloride is a dopamine receptor agonist with selectivity for the D3 receptor (Ki values are 3.9, 3.3, 0.5 and 3.9 nM for D2L, D2S, D3 and D4 receptors respectively). Pramipexole dihydrochloride exhibits negligable affinity for D1 and D5 receptors. Pramipexole has been found to have neuroprotective effects independent of its dopamine receptor agonism. It reduces mitochondrial reactive oxygen species (ROS) production and inhibits the activation of apoptotic pathways. Pramipexole displays activity in the treatment of Parkinson's disease (PD) and restless legs syndrome (RLS).
- CGS 15943
Catalog No.:BCC7157
CAS No.:104615-18-1
- Caffeic acid phenethyl ester
Catalog No.:BCN2695
CAS No.:104594-70-9
- p-Menthan-3-one
Catalog No.:BCN3850
CAS No.:10458-14-7
- CAY10603
Catalog No.:BCC5542
CAS No.:1045792-66-2
- EC 23
Catalog No.:BCC6097
CAS No.:104561-41-3
- Bayogenin 3-O-beta-D-glucopyranoside
Catalog No.:BCN7868
CAS No.:104513-86-2
- Testosterone acetate
Catalog No.:BCC9165
CAS No.:1045-69-8
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- 3'-Methylflavokawin
Catalog No.:BCN3990
CAS No.:1044743-35-2
- Typhaneoside
Catalog No.:BCN4994
CAS No.:104472-68-6
- L803-mts
Catalog No.:BCC5889
CAS No.:1043881-55-5
- RU-SKI 43
Catalog No.:BCC5441
CAS No.:1043797-53-0
- Pramipexole
Catalog No.:BCC4467
CAS No.:104632-26-0
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- H-Ile-NH2.HCl
Catalog No.:BCC2962
CAS No.:10466-56-5
- 7,3'-Di-O-methylorobol
Catalog No.:BCN6831
CAS No.:104668-88-4
- Iriflophenone 3-C-beta-D-glucopyranoside
Catalog No.:BCN1635
CAS No.:104669-02-5
- Lupiwighteone
Catalog No.:BCN4045
CAS No.:104691-86-3
- Ganoderic acid K
Catalog No.:BCN3039
CAS No.:104700-95-0
- Ganoderol B
Catalog No.:BCN5859
CAS No.:104700-96-1
- Ganoderol A
Catalog No.:BCN5860
CAS No.:104700-97-2
- Ganoderal A
Catalog No.:BCN2451
CAS No.:104700-98-3
- Boc-D-Glu(OtBu)-OH
Catalog No.:BCC3395
CAS No.:104719-63-3
Validated chiral liquid chromatographic method for the enantiomeric separation of Pramipexole dihydrochloride monohydrate.[Pubmed:16580170]
J Pharm Biomed Anal. 2006 Jun 16;41(4):1152-6.
A chiral liquid chromatographic method was developed for the enantiomeric resolution of Pramipexole dihydrochloride monohydrate, (S)-2-amino-4,5,6,7-tetra-hydro-6-(propylamino) benzothiazole dihydrochloride monohydrate, a dopamine agonist in bulk drugs. The enantiomers of Pramipexole dihydrochloride monohydrate were resolved on a Chiralpak AD (250 mm x 4.6 mm, 10 microm) column using a mobile phase system containing n-hexane:ethanol:diethylamine (70:30:0.1, v/v/v). The resolution between the enantiomers was found not less than eight. The presence of diethylamine in the mobile phase has played an important role in enhancing chromatographic efficiency and resolution between the enantiomers. The developed method was extensively validated and proved to be robust. The limit of detection and limit of quantification of (R)-enantiomer were found to be 300 and 900 ng/ml, respectively for 20 microl injection volume. The percentage recovery of (R)-enantiomer was ranged from 97.3 to 102.0 in bulk drug samples of Pramipexole dihydrochloride monohydrate. Pramipexole dihydrochloride monohydrate sample solution and mobile phase were found to be stable for at least 48 h. The proposed method was found to be suitable and accurate for the quantitative determination of (R)-enantiomer in bulk drugs.
Effects of a dopamine agonist on the pharmacodynamics of levodopa in Parkinson disease.[Pubmed:20065126]
Arch Neurol. 2010 Jan;67(1):27-32.
BACKGROUND: Treatment of Parkinson disease commonly includes levodopa and dopamine agonists; however, the interaction of these 2 drugs is poorly understood. OBJECTIVE: To examine the effects of a dopamine agonist on the motor response to levodopa. DESIGN: Double-blind, randomized, placebo-controlled, crossover clinical trial. SETTING: Ambulatory academic referral center. Patients Thirteen patients with idiopathic Parkinson disease taking levodopa and experiencing motor fluctuations and dyskinesia. INTERVENTIONS: Eligible individuals were randomly assigned to receive Pramipexole dihydrochloride or placebo for 4 weeks followed by a 2-hour intravenous levodopa infusion on consecutive days at 2 rates and with blinded assessments. They were then crossed over to the alternate oral therapy for 4 weeks followed by levodopa infusion and reassessment. MAIN OUTCOME MEASURES: Change in finger-tapping speed, measured using the area under the curve (AUC) for finger taps per minute across time; peak finger-tapping speed; duration of response; time to "ON" (defined as a 10% increase in finger-tapping speed above baseline); walking speed; and dyskinesia AUC. RESULTS: Pramipexole with levodopa infusion increased finger-tapping speed beyond the change in baseline by a mean (SE) of 170 (47.2) per minute x minutes (P = .006) and more than doubled the AUC for finger-tapping speed. Pramipexole increased peak finger-tapping speed by a mean (SE) of 18 (8.5) taps per minute (P = .02) and improved mean (SE) walking speed (15.9 [0.70] vs 18.9 [0.70] seconds, P = .004). Pramipexole prolonged duration of response after levodopa infusion and shortened time to ON. Pramipexole increased mean (SE) baseline dyskinesia scores (26.0 [5.85] vs 12.1 [5.85] points, P = .05) and peak dyskinesia scores with levodopa infusion. CONCLUSIONS: Pramipexole augmented the motor response to levodopa beyond a simple additive effect and increased the severity of levodopa-induced dyskinesia. When considering a combination of these therapies, an appropriate balance should be maintained regarding gain of motor function vs worsening of dyskinesia. Trial Registration clinicaltrials.gov Identifier: NCT00666653.
Pramipexole binding and activation of cloned and expressed dopamine D2, D3 and D4 receptors.[Pubmed:7664822]
Eur J Pharmacol. 1995 Jun 23;290(1):29-36.
Pramipexole (SND 919; 2-amino-4,5,6,7-tetrahydro-6-propylamino-benzthiazole-dihydrochlor ide) is a potent dopamine autoreceptor agonist. We have carried out an analysis of the binding affinities of dopamine D2L, D2S, D3, and D4 receptors for pramipexole using both [3H]pramipexole and [3H]spiperone as radioligands at cloned and heterologously expressed receptors. Studies were carried out using rat and human D2L, D2S and D3 receptors with equivalent results. When the binding of pramipexole to the high affinity, guanine nucleotide-sensitive state of each receptor was analyzed, pramipexole is most selective for D3 compared to D2 and D4 receptors. These results indicate a 5-fold selectivity of pramipexole for D3 receptors, while quinpirole and bromocriptine are non-selective or more D2/D4 receptor selective. Two measurements of receptor activation for dopamine D2, D3, and D4 receptors also show that pramipexole is most potent for activation of D3 receptors. The dopamine D3 receptor selectivity of pramipexole may explain the previously described properties of this drug, including its potent autoreceptor preference.


