Dexpramipexole dihydrochlorideNeuroprotective agent CAS# 104632-27-1 |
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- L-Stepholidine
Catalog No.:BCN2599
CAS No.:16562-13-3
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104632-27-1 | SDF | Download SDF |
| PubChem ID | 51052075 | Appearance | Powder |
| Formula | C10H19Cl2N3S | M.Wt | 284.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (351.80 mM) H2O : 100 mg/mL (351.80 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
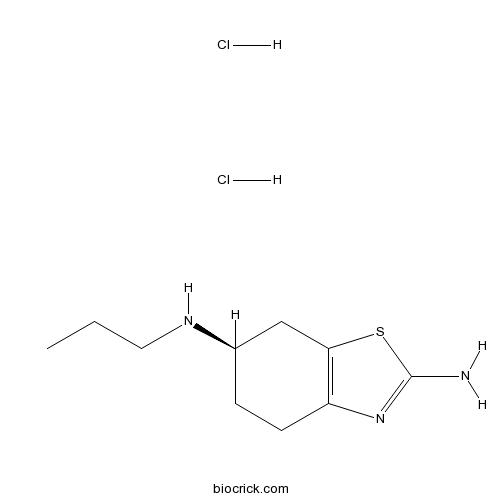
| Chemical Name | (6R)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine;dihydrochloride | ||
| SMILES | CCCNC1CCC2=C(C1)SC(=N2)N.Cl.Cl | ||
| Standard InChIKey | QMNWXHSYPXQFSK-XCUBXKJBSA-N | ||
| Standard InChI | InChI=1S/C10H17N3S.2ClH/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8;;/h7,12H,2-6H2,1H3,(H2,11,13);2*1H/t7-;;/m1../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dexpramipexole dihydrochloride Dilution Calculator

Dexpramipexole dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.518 mL | 17.5901 mL | 35.1803 mL | 70.3606 mL | 87.9507 mL |
| 5 mM | 0.7036 mL | 3.518 mL | 7.0361 mL | 14.0721 mL | 17.5901 mL |
| 10 mM | 0.3518 mL | 1.759 mL | 3.518 mL | 7.0361 mL | 8.7951 mL |
| 50 mM | 0.0704 mL | 0.3518 mL | 0.7036 mL | 1.4072 mL | 1.759 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3518 mL | 0.7036 mL | 0.8795 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dexpramipexole, also known as R-(+)-Pramipexole, is a neuroprotective agent and weak non-ergoline dopamine agonist. Dexpramipexole has been found to have neuroprotective effects and is being investigated for treatment of amyotrophic lateral sclerosis (ALS). Dexpramipexole reduces mitochondrial reactive oxygen species (ROS) production, inhibits the activation of apoptotic pathways, and increase cell survival in response to a variety of neurotoxins and β-amyloid neurotoxicity. Compared to the S-(-) isomer, Dexpramipexole has much lower dopamine agonist activity.
- Pramipexole
Catalog No.:BCC4467
CAS No.:104632-26-0
- Pramipexole dihydrochloride
Catalog No.:BCN2181
CAS No.:104632-25-9
- CGS 15943
Catalog No.:BCC7157
CAS No.:104615-18-1
- Caffeic acid phenethyl ester
Catalog No.:BCN2695
CAS No.:104594-70-9
- p-Menthan-3-one
Catalog No.:BCN3850
CAS No.:10458-14-7
- CAY10603
Catalog No.:BCC5542
CAS No.:1045792-66-2
- EC 23
Catalog No.:BCC6097
CAS No.:104561-41-3
- Bayogenin 3-O-beta-D-glucopyranoside
Catalog No.:BCN7868
CAS No.:104513-86-2
- Testosterone acetate
Catalog No.:BCC9165
CAS No.:1045-69-8
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- 3'-Methylflavokawin
Catalog No.:BCN3990
CAS No.:1044743-35-2
- Typhaneoside
Catalog No.:BCN4994
CAS No.:104472-68-6
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- H-Ile-NH2.HCl
Catalog No.:BCC2962
CAS No.:10466-56-5
- 7,3'-Di-O-methylorobol
Catalog No.:BCN6831
CAS No.:104668-88-4
- Iriflophenone 3-C-beta-D-glucopyranoside
Catalog No.:BCN1635
CAS No.:104669-02-5
- Lupiwighteone
Catalog No.:BCN4045
CAS No.:104691-86-3
- Ganoderic acid K
Catalog No.:BCN3039
CAS No.:104700-95-0
- Ganoderol B
Catalog No.:BCN5859
CAS No.:104700-96-1
- Ganoderol A
Catalog No.:BCN5860
CAS No.:104700-97-2
- Ganoderal A
Catalog No.:BCN2451
CAS No.:104700-98-3
- Boc-D-Glu(OtBu)-OH
Catalog No.:BCC3395
CAS No.:104719-63-3
- 8-Phenyloctanol
Catalog No.:BCC8791
CAS No.:10472-97-6
- 6-O-α-Maltosyl-β-cyclodextrin
Catalog No.:BCC8075
CAS No.:104723-60-6
Solid Microneedles for Transdermal Delivery of Amantadine Hydrochloride and Pramipexole Dihydrochloride.[Pubmed:26426039]
Pharmaceutics. 2015 Sep 28;7(4):379-96.
The aim of this project was to study the influence of microneedles on transdermal delivery of amantadine hydrochloride and pramipexole dihydrochloride across porcine ear skin in vitro. Microchannel visualization studies were carried out and characterization of the microchannel depth was performed using confocal laser scanning microscopy (CLSM) to demonstrate microchannel formation following microneedle roller application. We also report, for the first time, the use of TA.XT Plus Texture Analyzer to characterize burst force in pig skin for transdermal drug delivery experiments. This is the force required to rupture pig skin. The mean passive flux of amantadine hydrochloride, determined using a developed LC-MS/MS technique, was 22.38 +/- 4.73 microg/cm(2)/h, while the mean flux following the use of a stainless steel microneedle roller was 49.04 +/- 19.77 microg/cm(2)/h. The mean passive flux of pramipexole dihydrochloride was 134.83 +/- 13.66 microg/cm(2)/h, while the flux following the use of a stainless steel microneedle roller was 134.04 +/- 0.98 microg/cm(2)/h. For both drugs, the difference in flux values following the use of solid stainless steel microneedle roller was not statistically significantly (p > 0.05). Statistical analysis was carried out using the Mann-Whitney Rank sum test.
New functional activity of aripiprazole revealed: Robust antagonism of D2 dopamine receptor-stimulated Gbetagamma signaling.[Pubmed:25449598]
Biochem Pharmacol. 2015 Jan 1;93(1):85-91.
The dopamine D2 receptor (DRD2) is a G protein-coupled receptor (GPCR) that is generally considered to be a primary target in the treatment of schizophrenia. First generation antipsychotic drugs (e.g. haloperidol) are antagonists of the DRD2, while second generation antipsychotic drugs (e.g. olanzapine) antagonize DRD2 and 5HT2A receptors. Notably, both these classes of drugs may cause side effects associated with D2 receptor antagonism (e.g. hyperprolactemia and extrapyramidal symptoms). The novel, "third generation" antipsychotic drug, aripiprazole is also used to treat schizophrenia, with the remarkable advantage that its tendency to cause extrapyramidal symptoms is minimal. Aripiprazole is considered a partial agonist of the DRD2, but it also has partial agonist/antagonist activity for other GPCRs. Further, aripiprazole has been reported to have a unique activity profile in functional assays with the DRD2. In the present study the molecular pharmacology of aripiprazole was further examined in HEK cell models stably expressing the DRD2 and specific isoforms of adenylyl cyclase to assess functional responses of Galpha and Gbetagamma subunits. Additional studies examined the activity of aripiprazole in DRD2-mediated heterologous sensitization of adenylyl cyclase and cell-based dynamic mass redistribution (DMR). Aripiprazole displayed a unique functional profile for modulation of G proteins, being a partial agonist for Galphai/o and a robust antagonist for Gbetagamma signaling. Additionally, aripiprazole was a weak partial agonist for both heterologous sensitization and dynamic mass redistribution.
Safety and Tolerability of R(+) Pramipexole in Mild-to-Moderate Alzheimer's Disease.[Pubmed:26682692]
J Alzheimers Dis. 2016;49(4):1179-87.
Alzheimer's disease (AD) is an aging-related, degenerative brain disease of adults. Most ( approximately 95%) of AD occurs sporadically and is associated with early-appearing deficits in brain regional glucose uptake, changes in cerebrospinal fluid (CSF) AD-related proteins, regional brain atrophy, and oxidative stress damage. We treated mild-moderate AD individuals with R(+)-pramipexole-dihydrochloride (R(+)PPX), a neuroprotective, lipophilic-cation, free-radical scavenger that accumulates into brain and mitochondria. 19 subjects took R(+)PPX twice a day in increasing daily doses up to 300 mg/day under a physician-sponsor IND (60,948, JPB), IRB-approved protocol and quarterly external safety committee monitoring. 15 persons finished and contributed baseline and post-treatment serum, lumbar spinal fluid, brain 18F-2DG PET scans, and ADAS-Cog scores. ADAS-Cog scores did not change (n = 1), improved (n = 2), declined 1-3 points (n = 5), or declined 4-13 points (n = 8) over 6 months of R(+)PPX treatment. Serum PPX levels were not related to changes in ADAS-Cog scores. Fasting AM serum PPX levels at 6 months varied considerably across subjects and correlated strongly with CSF [PPX] (r = 0.97, p < 0.0001). CSF [PPX] was not related to CSF [Abeta(42)], [Tau], or [P-Tau]. Regional 18F-2DG measures of brain glucose uptake demonstrated a 3-6% decline during R(+)PPX treatment. 56 mild-moderate adverse events occurred, 26 probably/definitely related to R(+)PPX use, with 4 withdrawals. R(+)PPX was generally well-tolerated and entered brain extracellular space linearly. Further studies of R(+)PPX in AD should include a detailed pharmacokinetic study of peak and trough serum [PPX] variations among subjects prior to planning any larger studies that would be needed to determine efficacy in altering disease progression.
[Feeding difficulty and developmental delay for 8 months and nystagmus for 4 months in an infant].[Pubmed:28100326]
Zhongguo Dang Dai Er Ke Za Zhi. 2017 Jan;19(1):68-72.
Aromatic L-amino acid decarboxylase (AADC) deficiency is a rare autosomal recessive hereditary disease and is a congenital metabolic disorder of neurotransmitter biosynthesis. It is mainly manifested as hypotonia, oculogyric crisis, autonomic dysfunction, and developmental delay. This article reports a boy manifested as delayed motor development, hypotonia, and oculogyric crisis. Gene screening for metabolic disorders revealed new compound heterozygous mutations, c.1063dupA (p.I355fs) and c.250A>C (p.S84R), in the exon of DDC gene. The boy had a significant increase in 3-O-methyldopa as measured by dried blood spot. Therefore, he was diagnosed with AADC deficiency. After treatment with the dopamine receptor agonist pramipexole dihydrochloride, the catechol-O-methyltransferase inhibitor entacapone, and vitamin B6, the boy showed mild improvements in hypotonia, blepharoptosis, and oculogyric crisis. Clinical physicians should enhance their ability for identifying AADC deficiency, so as to facilitate early diagnosis and treatment of this disorder. Genetic counseling for birth health and prenatal diagnosis should be performed for parents in need.
In vivo dopamine agonist properties of rotigotine: Role of D1 and D2 receptors.[Pubmed:27343381]
Eur J Pharmacol. 2016 Oct 5;788:183-191.
Rotigotine acts in vitro as a full agonist of dopamine D1 receptors at concentrations almost superimposable to those at which it acts on D2 receptors. However in vivo evidence of the differences between the agonist activity of rotigotine at D1 receptors from that on the D2 receptors has not been provided yet. In order to test the ability of rotigotine to stimulate dopamine D1 and D2 receptors in vivo, we studied the effect of SCH39166 and eticlopride, selective dopamine D1 and D2/D3 receptor antagonists respectively, on rotigotine-induced contralateral turning behavior in 6-hydroxydopamine lesioned rats. Furthermore, the expression of the immediate-early gene c-fos in the caudate-putamen, was evaluated. As a comparison, we tested the D2/D3 agonist pramipexole. In primed rats, rotigotine (0.035, 0.1 and 0.35mg/kg) induced dose-dependent contralateral turning. Turning induced by 0.1mg/kg of rotigotine was reduced by pretreatment with the D1 antagonist SCH39166 and the D2 antagonist eticlopride. In drug-naive rats, rotigotine was less effective in eliciting turning but SCH39166 still reduced turning induced by rotigotine (0.35mg/kg). Pramipexole induced contralateral turning only in primed rats. SCH39166 potentiated and eticlopride abolished pramipexole-induced turning. Rotigotine induced Fos expression in the caudate-putamen and SCH39166 completely blocked it. Pramipexole failed to induce Fos. These results indicate that rotigotine acts in vivo as an agonist of D1 and D2 receptors while pramipexole is devoid of D1 activity in vivo. Given their differing DA receptor profiles, rotigotine and pramipexole might differ in their spectrum of application to the therapy of Parkinson's disease.


