p-Menthan-3-oneCAS# 10458-14-7 |
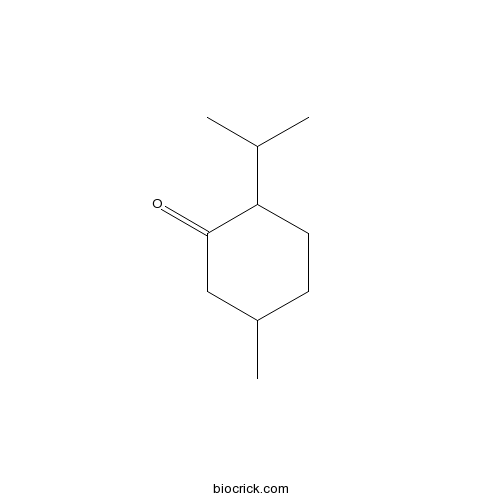
- (+)-Menthone
Catalog No.:BCC9239
CAS No.:89-80-5
- (-)-Menthone
Catalog No.:BCN9070
CAS No.:14073-97-3
- Isomenthone
Catalog No.:BCN0328
CAS No.:491-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10458-14-7 | SDF | Download SDF |
| PubChem ID | 6986 | Appearance | Oil |
| Formula | C10H18O | M.Wt | 154.3 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-methyl-2-propan-2-ylcyclohexan-1-one | ||
| SMILES | CC1CCC(C(=O)C1)C(C)C | ||
| Standard InChIKey | NFLGAXVYCFJBMK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H18O/c1-7(2)9-5-4-8(3)6-10(9)11/h7-9H,4-6H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Menthone has anti-inflammary activity, it can suppress the lipopolysaccharide (LPS)-induced proinflammatory cytokines, interleukin-1beta (IL-1beta) and tumor necrosis factor-alpha (TNF-alpha), as well as nuclear factor kappaB (NF-kappaB) activity induced by LPS and other inflammatory agents. Menthone and menthol enhances the skin permeability by disordering the ordered organization of SC lipids and extracts part of SC lipids. |
| Targets | IL Receptor | TNF-α | NF-kB |
| In vitro | Inhibition of lipopolysaccharide-induced interleukin-1beta and tumor necrosis factor-alpha production by menthone through nuclear factor-kappaB signaling pathway in HaCat cells.[Pubmed: 18935911]Chin J Physiol. 2008 Jun 30;51(3):160-6.Menthone, the Chinese old remedy extracted from genus Mentha, has been widely used as a cooling agent, a counterirritant for pain relief, and for the treatment of pruritus. However, its detail mechanisms for interfering inflammatory reaction remain unknown. |
| In vivo | Research on choleretic effect of menthol, menthone, pluegone, isomenthone, and limonene in DanShu capsule.[Pubmed: 25499726]Int Immunopharmacol. 2015 Feb;24(2):191-7.Danshu capsule (DSC) is a medicinal compound in traditional Chinese medicine (TCM). It is commonly used for the treatment of acute & chronic cholecystitis as well as choleithiasis. |
| Kinase Assay | Effect of menthone and related compounds on skin permeation of drugs with different lipophilicity and molecular organization of stratum corneum lipids.[Pubmed: 25684238]Pharm Dev Technol. 2015 Feb 16:1-10.The objective of this article was to investigate the enhancing effect of Menthone, menthol and pulegone on the transdermal absorption of drugs with different lipophilicity and probe their mechanisms of action at molecular level. |

p-Menthan-3-one Dilution Calculator

p-Menthan-3-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4809 mL | 32.4044 mL | 64.8088 mL | 129.6176 mL | 162.022 mL |
| 5 mM | 1.2962 mL | 6.4809 mL | 12.9618 mL | 25.9235 mL | 32.4044 mL |
| 10 mM | 0.6481 mL | 3.2404 mL | 6.4809 mL | 12.9618 mL | 16.2022 mL |
| 50 mM | 0.1296 mL | 0.6481 mL | 1.2962 mL | 2.5924 mL | 3.2404 mL |
| 100 mM | 0.0648 mL | 0.324 mL | 0.6481 mL | 1.2962 mL | 1.6202 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CAY10603
Catalog No.:BCC5542
CAS No.:1045792-66-2
- EC 23
Catalog No.:BCC6097
CAS No.:104561-41-3
- Bayogenin 3-O-beta-D-glucopyranoside
Catalog No.:BCN7868
CAS No.:104513-86-2
- Testosterone acetate
Catalog No.:BCC9165
CAS No.:1045-69-8
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- 3'-Methylflavokawin
Catalog No.:BCN3990
CAS No.:1044743-35-2
- Typhaneoside
Catalog No.:BCN4994
CAS No.:104472-68-6
- L803-mts
Catalog No.:BCC5889
CAS No.:1043881-55-5
- RU-SKI 43
Catalog No.:BCC5441
CAS No.:1043797-53-0
- Tetrahydroxysqualene
Catalog No.:BCN5858
CAS No.:1043629-23-7
- Bisoprolol fumarate
Catalog No.:BCC4344
CAS No.:104344-23-2
- IRAK inhibitor 6
Catalog No.:BCC1658
CAS No.:1042672-97-8
- Caffeic acid phenethyl ester
Catalog No.:BCN2695
CAS No.:104594-70-9
- CGS 15943
Catalog No.:BCC7157
CAS No.:104615-18-1
- Pramipexole dihydrochloride
Catalog No.:BCN2181
CAS No.:104632-25-9
- Pramipexole
Catalog No.:BCC4467
CAS No.:104632-26-0
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- H-Ile-NH2.HCl
Catalog No.:BCC2962
CAS No.:10466-56-5
- 7,3'-Di-O-methylorobol
Catalog No.:BCN6831
CAS No.:104668-88-4
- Iriflophenone 3-C-beta-D-glucopyranoside
Catalog No.:BCN1635
CAS No.:104669-02-5
- Lupiwighteone
Catalog No.:BCN4045
CAS No.:104691-86-3
- Ganoderic acid K
Catalog No.:BCN3039
CAS No.:104700-95-0
- Ganoderol B
Catalog No.:BCN5859
CAS No.:104700-96-1
Research on choleretic effect of menthol, menthone, pluegone, isomenthone, and limonene in DanShu capsule.[Pubmed:25499726]
Int Immunopharmacol. 2015 Feb;24(2):191-197.
Danshu capsule (DSC) is a medicinal compound in traditional Chinese medicine (TCM). It is commonly used for the treatment of acute & chronic cholecystitis as well as choleithiasis. To study its choleretic effect, healthy rats were randomly divided into DSC high (DSCH, 900mg/kg), medium (DSCM, 450mg/kg), and low (DSCL, 225mg/kg) group, Xiaoyan Lidan tablet (XYLDT, 750mg/kg), and saline group. The bile was collected for 1h after 20-minute stabilization as the base level, and at 1h, 2h, 3h, and 4h after drug administration, respectively. Bile volume, total cholesterol, and total bile acid were measured at each time point. The results revealed that DSC significantly stimulated bile secretion, decreased total cholesterol level and increased total bile acid level. Therefore, it had choleretic effects. To identify the active components contributing to its choleretic effects, five major constituents which are menthol (39.33mg/kg), menthone (18.02mg/kg), isomenthone (8.18mg/kg), pluegone (3.31mg/kg), and limonene (4.39mg/kg) were tested on our rat model. The results showed that menthol and limonene could promote bile secretion when compared to DSC treatment (p > 0.05); Menthol, menthol and limonene could significantly decrease total cholesterol level (p<0.05 or p<0.01) as well as increase total bile acid level (p<0.05 or p<0.01); Isomenthone, as a isomer of menthone, existed slightly choleretic effects; Pluegone had no obvious role in bile acid efflux. These findings indicated that the choleretic effects of DSC may be attributed mainly to its three major constituents: menthol, menthone and limonene.
Inhibition of lipopolysaccharide-induced interleukin-1beta and tumor necrosis factor-alpha production by menthone through nuclear factor-kappaB signaling pathway in HaCat cells.[Pubmed:18935911]
Chin J Physiol. 2008 Jun 30;51(3):160-6.
Menthone, the Chinese old remedy extracted from genus Mentha, has been widely used as a cooling agent, a counterirritant for pain relief, and for the treatment of pruritus. However, its detail mechanisms for interfering inflammatory reaction remain unknown. In this study, we found that menthone can suppress the lipopolysaccharide (LPS)-induced proinflammatory cytokines, interleukin-1beta (IL-1beta) and tumor necrosis factor-alpha (TNF-alpha), as well as nuclear factor kappaB (NF-kappaB) activity induced by LPS and other inflammatory agents, including 12-O-tetradecanoylphorbol-13-acetate, hydrogen peroxide, okadaic acid, and ceramide. Furthermore, our data also demonstrated that the translocation of NF-kappaB activated by LPS into the nucleus was suppressed by menthone, and I-kappaB and beta-transducin repeat containing protein (beta-TrCP) were both involved in this suppression. To sum up, this study has provided molecular evidence for menthone effect on the LPS-induced cytokine production, NF-kappaB activation, and the involvement of I-kappaB and beta-TrCP.
Effect of menthone and related compounds on skin permeation of drugs with different lipophilicity and molecular organization of stratum corneum lipids.[Pubmed:25684238]
Pharm Dev Technol. 2016;21(4):389-98.
The objective of this article was to investigate the enhancing effect of menthone, menthol and pulegone on the transdermal absorption of drugs with different lipophilicity and probe their mechanisms of action at molecular level. Five model drugs, namely osthole, tetramethylpyrazine, ferulic acid, puerarin and geniposide, which were selected based on their lipophilicity denoted by logKo/w, were tested using in vitro permeation studies in which Franz diffusion cells and rat skin were employed. Infrared spectroscopy and molecular dynamic simulation were used to investigate the effect of these enhancers on the stratum corneum (SC) lipids, respectively. Three compounds could effectively promote the transdermal absorption of drugs with different lipophilicity, and the overall promoting capacities were in the following increasing order: pulegone < menthol < menthone. The penetration enhancement ratio was roughly in parabolic curve relationships with the drug lipophilicity after treatment with menthol or menthone, while the penetration enhancement effect of pulegone hardly changed with the alteration of the drug lipophilicity. The molecular mechanism studies suggested that menthone and menthol enhanced the skin permeability by disordering the ordered organization of SC lipids and extracted part of SC lipids, while pulegone appeared to promote drug transport across the skin only by extracting part of SC lipids.


