Bisoprolol fumarateCAS# 104344-23-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104344-23-2 | SDF | Download SDF |
| PubChem ID | 5281064 | Appearance | Powder |
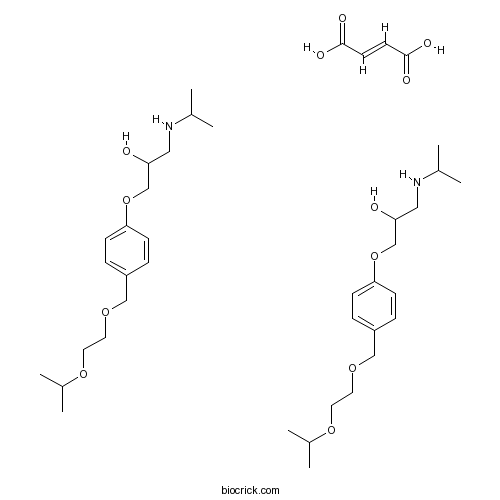
| Formula | C22H35NO8 | M.Wt | 441.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (65.19 mM) H2O : 20 mg/mL (26.08 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (±)-1-[4-[[2-(1-Methylethoxy)ethoxy) | ||
| SMILES | CC(C)NCC(O)COc1ccc(COCCOC(C)C)cc1.CC(C)NCC(O)COc2ccc(COCCOC(C)C)cc2.OC(=O)C=CC(O)=O | ||
| Standard InChIKey | VMDFASMUILANOL-WXXKFALUSA-N | ||
| Standard InChI | InChI=1S/2C18H31NO4.C4H4O4/c2*1-14(2)19-11-17(20)13-23-18-7-5-16(6-8-18)12-21-9-10-22-15(3)4;5-3(6)1-2-4(7)8/h2*5-8,14-15,17,19-20H,9-13H2,1-4H3;1-2H,(H,5,6)(H,7,8)/b;;2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A selective β1-adrenergic antagonist. Has a Kd of 2-3 nM at the β1 receptor and a β1/β2 selectivity of approximately 100-fold. |

Bisoprolol fumarate Dilution Calculator

Bisoprolol fumarate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2649 mL | 11.3245 mL | 22.649 mL | 45.2981 mL | 56.6226 mL |
| 5 mM | 0.453 mL | 2.2649 mL | 4.5298 mL | 9.0596 mL | 11.3245 mL |
| 10 mM | 0.2265 mL | 1.1325 mL | 2.2649 mL | 4.5298 mL | 5.6623 mL |
| 50 mM | 0.0453 mL | 0.2265 mL | 0.453 mL | 0.906 mL | 1.1325 mL |
| 100 mM | 0.0226 mL | 0.1132 mL | 0.2265 mL | 0.453 mL | 0.5662 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bisoprolol fumarate
- IRAK inhibitor 6
Catalog No.:BCC1658
CAS No.:1042672-97-8
- Famciclovir
Catalog No.:BCC4780
CAS No.:104227-87-4
- IRAK inhibitor 1
Catalog No.:BCC1654
CAS No.:1042224-63-4
- Yunnandaphninine G
Catalog No.:BCN5857
CAS No.:1042143-83-8
- Estriol 3,17-dihexanoate
Catalog No.:BCN2238
CAS No.:104202-96-2
- 10-Nitro-camptothecin
Catalog No.:BCN2581
CAS No.:104195-61-1
- Stanozolol
Catalog No.:BCC9154
CAS No.:10418-03-8
- Alpinoid D
Catalog No.:BCN3593
CAS No.:1041740-13-9
- MitoPY1
Catalog No.:BCC6177
CAS No.:1041634-69-8
- Kuguaglycoside C
Catalog No.:BCN3276
CAS No.:1041631-93-9
- Peramivir Trihydrate
Catalog No.:BCC4956
CAS No.:1041434-82-5
- Eldecalcitol
Catalog No.:BCC1548
CAS No.:104121-92-8
- Tetrahydroxysqualene
Catalog No.:BCN5858
CAS No.:1043629-23-7
- RU-SKI 43
Catalog No.:BCC5441
CAS No.:1043797-53-0
- L803-mts
Catalog No.:BCC5889
CAS No.:1043881-55-5
- Typhaneoside
Catalog No.:BCN4994
CAS No.:104472-68-6
- 3'-Methylflavokawin
Catalog No.:BCN3990
CAS No.:1044743-35-2
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- Testosterone acetate
Catalog No.:BCC9165
CAS No.:1045-69-8
- Bayogenin 3-O-beta-D-glucopyranoside
Catalog No.:BCN7868
CAS No.:104513-86-2
- EC 23
Catalog No.:BCC6097
CAS No.:104561-41-3
- CAY10603
Catalog No.:BCC5542
CAS No.:1045792-66-2
- p-Menthan-3-one
Catalog No.:BCN3850
CAS No.:10458-14-7
- Caffeic acid phenethyl ester
Catalog No.:BCN2695
CAS No.:104594-70-9
Spectrophotometric method for estimation of bisoprolol fumarate in tablets.[Pubmed:25076731]
Rev Med Chir Soc Med Nat Iasi. 2014 Apr-Jun;118(2):558-63.
UNLABELLED: Bisoprolol fumarate is prescribed for the treatment of hypertension and angina pectoris. AIM: The purpose of this study was to develop a simple, sensitive, accurate, and reproducible method for estimation of Bisoprolol fumarate in tablets. MATERIAL AND METHODS: The proposed method was based on a yellow colored complex formed with tropaeolin 00, extractable in dichloromethane with maximum absorbance at 412 nm. The method was validated statistically. RESULTS: The linearity domain was observed in the concentration of 5-30 microg/ml. The recovery studies confirmed the accuracy of the proposed method. CONCLUSIONS: The proposed method can be applied for the routine analysis of bisoprolol from formulations.
IN VITRO TESTING OF XANTHAN/LIGNIN HYDROGELS AS CARRIERS FOR CONTROLLED DELIVERY OF BISOPROLOL FUMARATE.[Pubmed:26793868]
Rev Med Chir Soc Med Nat Iasi. 2015 Oct-Dec;119(4):1189-94.
AIM: To develop sustained release matrix tablets based on xanthan as highly water-soluble, cost-effective, non-toxic, easily available, and suitable hydrophilic systems. MATERIAL AND METHODS: Xanthan and lignin epoxy-modified resin (LER) mixture were crosslinked using epichlorohydrin as crosslinking agent leading to superabsorbent hydrogels with high swelling rate in aqueous mediums. RESULTS AND CONCLUSIONS: These hydrogels were tested as carries by the loading/delivery behaviour of Bisoprolol fumarate in physiological conditions and based on the obtained results these hydrogels may show interest for application in medical and pharmaceutical areas. The amount of drug loaded in polymer networks was found to be ranging between 14.4% and 19.2%. Drug release was retarded and the release mechanism of the active principle was found to depend on matrix composition.
Preparation of bisoprolol fumarate nasal spray and its nasal delivery in rats.[Pubmed:26639509]
Pak J Pharm Sci. 2015 Nov;28(6):2173-8.
The aim of the present work was to prepare a nasal spray of Bisoprolol fumarate (BF). The Pharmacokinetics and relative bioavailability of the BF nasal formulation were evaluated in Wistar rats. The BF nasal spray after administration exhibited very fast absorption and higher plasma drug concentration. The maximum plasma concentration (C(max)) and the time to reach it (T(max)) were 409.5 ng/ml and 3.6 min for the BF nasal spray, 39.4 ng/ml and 26.7 min for the drug solution, respectively. The bioavailability of the BF nasal spray was greater than 1500.0%. Meantime, the effect of the BF nasal spray on nasal mucociliary movement was also studied with a toad palate model. The BF nasal preparation showed minor ciliotoxicity, but the adverse effect was temporary and reversible.
Application of HPLC to assess the compatibility of bisoprolol fumarate with selected excipients in mixtures by isothermal stress testing.[Pubmed:26142745]
Ann Pharm Fr. 2015 Nov;73(6):442-51.
In the present study, the compatibility between Bisoprolol fumarate and selected excipients (ascorbic acid, citric acid anhydrous, butylated hydroxyanisole, polyvinylpyrrolidone, glycerol, mannitol and sorbitol) in mixtures (1:10 ratio of drug and excipient) was investigated by subjecting the samples to isothermal stress conditions (90 degrees C for 48 h). A new HPLC method was developed, validated and employed for determining the drug content of the stressed compatibility samples. Results of HPLC revealed that major degradation of Bisoprolol fumarate was observed with butylated hydroxyanisole (89.4%), citric acid anhydrous (89%), mannitol (77%) and glycerol (61.9%).
Studies on the receptor profile of bisoprolol.[Pubmed:2870719]
Arzneimittelforschung. 1986 Feb;36(2):197-200.
The in vitro binding affinity of (+/-)-1-[4-(2-isopropoxyethoxymethyl)-phenoxy]-3-isopropylamino-2- propranol hemifumarate (bisoprolol, EMD 33 512) to beta 1-, beta 2-, alpha 1-, alpha 2-, D1-, D2-, 5-HT2- and muscarinic cholinergic receptors of rat was compared with that of atenolol, betaxolol and propranolol. Bisoprolol showed a high specific binding affinity to beta 1-adrenoceptors (heart) and a low specific binding affinity to beta 2-adrenoceptors (lung). The beta 1-selectivity of bisoprolol (beta 2/beta 1 = 34.7) proved to be higher than that of atenolol (8.7) and betaxolol (12.5). Propranolol (0.59) was non-selective as expected. Bisoprolol and atenolol exhibited no remarkable binding affinity to alpha 1-, alpha 2-, D1-, D2-, 5-HT2- and muscarinic cholinergic receptors at concentrations up to 1 X 10(-4) mol/l. For betaxolol binding affinities for alpha 2-, D2- and 5-HT2-receptors were found with IC50 values ranging between 2 X 10(-5) and 7 X 10(-5) mol/l. For propranolol binding affinities for alpha 1-, alpha 2-, D1-, D2- and 5-HT2-receptors were found with IC50 values ranging between 2 X 10(-6) and 9 X 10(-5) mol/l.
Direct labelling of myocardial beta 1-adrenoceptors. Comparison of binding affinity of 3H-(-)-bisoprolol with its blocking potency.[Pubmed:2866449]
Naunyn Schmiedebergs Arch Pharmacol. 1985 Oct;331(1):27-39.
A radioligand that selectively labels beta 1-adrenoceptors, 3H-(-)-bisoprolol (3H-BIS), is introduced. The binding properties of 3H-BIS to membrane particles of kitten heart are compared with the blocking properties of (-)-bisoprolol against stimulant effects of (-)-adrenaline and (-)-noradrenaline in heart preparations of kitten and guinea pig. 1. On kitten heart tissues (-)-bisoprolol antagonized the positive chronotropic and inotropic effects of catecholamines competitively. The effects of (-)-adrenaline were antagonized considerably less by (-)-bisoprolol than the corresponding effects of (-)-noradrenaline on sinoatrial pacemakers. The antagonism was nearly the same against both (-)-adrenaline and (-)-noradrenaline in left atria and papillary muscles. The data were analyzed with a model for 2-receptor subtypes by non-linear regression. Equilibrium dissociation constants KB (mol/l; -log KB = pKB) for a high-affinity beta 1-adrenoceptor of 8.8 and for a low-affinity beta 2-adrenoceptor of 7.0 were estimated in the three classes of tissues. In kitten sinoatrial pacemaker beta 1-adrenoceptors contribute 76% to the stimulus induced by (-)-adrenaline and 97% to the stimulus induced by (-)-noradrenaline. In ventricle and left atrium beta 1-adrenoceptors contribute 97-99% and 100% to the stimulus caused by (-)-adrenaline and (-)-noradrenaline, respectively. 2. Both 3H-BIS and unlabelled (-)-bisoprolol caused competitive blockade of the positive chronotropic effects of (-)-noradrenaline in guinea-pig right atria. pKB-values of 8.7 were estimated for both unlabelled and tritiated (-)-bisoprolol. The positive chronotropic effects of (-)-adrenaline were antagonized considerably less by (-)-bisoprolol than those of (-)-noradrenaline in guinea-pig atria. In the presence of low concentrations of beta 2-selective ICI 118,551, which did not antagonize beta 1-adrenoceptor mediated effects, (-)-bisoprolol antagonized positive chronotropic effects of (-)-adrenaline to the same extent as those of (-)-noradrenaline. The results are consistent with the concept of a significant role of sinoatrial beta 2-adrenoceptors of guinea pig for the effects of (-)-adrenaline but not for those of (-)-noradrenaline. 3. 3H-BIS associated and dissociated quickly with and from ventricular beta 1-adrenoceptors. A koff of 1.0 min-1 was estimated. An equilibrium dissociation constant pKL* of 8.2 for 3H-BIS was estimated from saturation binding.(ABSTRACT TRUNCATED AT 400 WORDS)


