UC 112IAP inhibitor CAS# 383392-66-3 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 383392-66-3 | SDF | Download SDF |
| PubChem ID | 3426979 | Appearance | Powder |
| Formula | C22H24N2O2 | M.Wt | 348.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
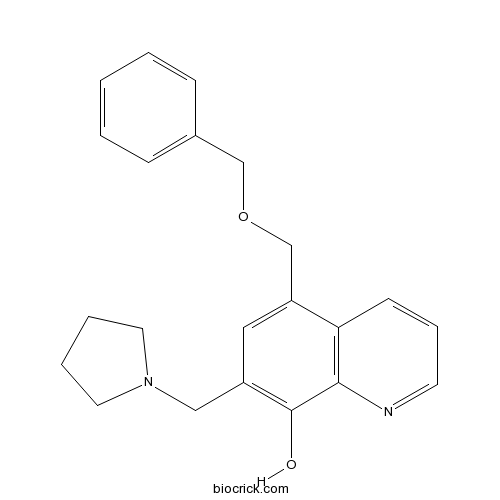
| Chemical Name | 5-(phenylmethoxymethyl)-7-(pyrrolidin-1-ylmethyl)quinolin-8-ol | ||
| SMILES | C1CCN(C1)CC2=C(C3=C(C=CC=N3)C(=C2)COCC4=CC=CC=C4)O | ||
| Standard InChIKey | LTGLGIQQZXSLLF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H24N2O2/c25-22-18(14-24-11-4-5-12-24)13-19(20-9-6-10-23-21(20)22)16-26-15-17-7-2-1-3-8-17/h1-3,6-10,13,25H,4-5,11-12,14-16H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | IAP inhibitor (IC50 values from 0.7 - 3.4 μM); inhibits cell growth in multiple cancer cell lines and in a melanoma xenograft model in vivo. Also suppresses X-linked inhibitor of apoptosis protein (XIAP) and survivin levels. Inhibits the growth of P-glycoproteins and activates caspase-3/7 and caspase-9. |

UC 112 Dilution Calculator

UC 112 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8699 mL | 14.3497 mL | 28.6993 mL | 57.3987 mL | 71.7484 mL |
| 5 mM | 0.574 mL | 2.8699 mL | 5.7399 mL | 11.4797 mL | 14.3497 mL |
| 10 mM | 0.287 mL | 1.435 mL | 2.8699 mL | 5.7399 mL | 7.1748 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.574 mL | 1.148 mL | 1.435 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.574 mL | 0.7175 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
UC-112[5-((benzyloxy)methyl)-7-(pyrrolidin-1-ylmethyl)quinolin-8-ol], is inhibitor of inhibitor of apoptosis protein (IAP).[1]
Inhibitor of apoptosis protein (IAP) family is overexpressed in most human cancer cells, but nearly isn’t expressed in adult differentiated tissues. Inhibitor of apoptosis (IAP) proteins are widely considered as promising drug-resistant tumors drug targets. [1,2]
UC-112 strongly activates caspase-3/7 and caspase-9 activities, and selectively reduce surviving level at a concentration as low as 1 mM. Coincubation of UC-112 with a known proteasome inhibitor Z-Leu-Leu-Leu-CHO (MG-132) rescued survivin inhibition. As a single agent, UC-112 strongly inhibits tumor growth and reduces both X chromosomelinked IAP and survivin levels in an A375 human melanoma xenograft model in vivo.[1]
UC-112 inhibits tumor cell growth in several cancer cell lines in vitro and suppresses melanoma tumor growth in vivo. Mechanistic studies indicated that UC-112 selectively inhibits survivin expression and induces strong cancer cell apoptosis. UC-112 could efficiently inhibit tumor cell proliferation by down regulating the level of IAPs, especially survivin protein.[1,2]
References:
[1] Wang J, Li W. Discovery of novel second mitochondria-derived activator of caspase mimetics as selective inhibitor of apoptosis protein inhibitors. J Pharmacol Exp Ther. 2014 May;349(2):319-29.
[2] Xiao M, Wang J, Lin Z, etal. , Design, Synthesis and Structure-Activity Relationship Studies of Novel Survivin Inhibitors with Potent Anti-Proliferative Properties. PLoS One. 2015 Jun 12;10(6):e0129807.
- Homovanillic Acid Sulfate
Catalog No.:BCN2226
CAS No.:38339-06-9
- 3-O-beta-D-Glucopyranosylplatycodigenin
Catalog No.:BCN7832
CAS No.:38337-25-6
- YE 120
Catalog No.:BCC6188
CAS No.:383124-82-1
- Minoxidil
Catalog No.:BCC4297
CAS No.:38304-91-5
- Naringenin trimethyl ether
Catalog No.:BCN5437
CAS No.:38302-15-7
- Burchellin
Catalog No.:BCN6676
CAS No.:38276-59-4
- Glucose-conjugated MGMT inhibitor
Catalog No.:BCC1597
CAS No.:382607-78-5
- Malonomicin
Catalog No.:BCN1844
CAS No.:38249-71-7
- 20(R)-Ginsenoside Rg3
Catalog No.:BCN5018
CAS No.:38243-03-7
- beta-Amyrenonol
Catalog No.:BCN5436
CAS No.:38242-02-3
- Enhydrin chlorohydrin
Catalog No.:BCN4639
CAS No.:38230-99-8
- Anhydroicaritin
Catalog No.:BCN5351
CAS No.:38226-86-7
- Neuropeptide W-23 (human)
Catalog No.:BCC5961
CAS No.:383415-79-0
- Taxinine
Catalog No.:BCN6944
CAS No.:3835-52-7
- 3',4'-Anhydrovinblastine
Catalog No.:BCN2392
CAS No.:38390-45-3
- NSC 663284
Catalog No.:BCC7199
CAS No.:383907-43-5
- Caudatin
Catalog No.:BCN5810
CAS No.:38395-02-7
- Ganaxolone
Catalog No.:BCC7397
CAS No.:38398-32-2
- Aloenin
Catalog No.:BCN8438
CAS No.:38412-46-3
- Altechromone A
Catalog No.:BCN7422
CAS No.:38412-47-4
- 3,4,4',7-Tetrahydroxyflavan
Catalog No.:BCN5438
CAS No.:38412-82-7
- Sclareol glycol
Catalog No.:BCN7007
CAS No.:38419-75-9
- H-Lys-OEt .2HCl
Catalog No.:BCC2980
CAS No.:3844-53-9
- Asatone
Catalog No.:BCN7761
CAS No.:38451-63-7
Commentary on "Robot-assisted laparoscopic vs open radical cystectomy: Comparison of complications and perioperative oncological outcomes in 200 patients." Kader AK, Richards KA, Krane LS, Pettus JA, Smith JJ, Hemal AK, Division of Urology, UC San Diego Health System, San Diego, CA.: BJU Int 2013; 112(4):E290-4. doi:10.1111/bju.12167. [Epub 2013 Jul 1].[Pubmed:25488382]
Urol Oncol. 2014 Nov;32(8):1348.
OBJECTIVE: To compare perioperative morbidity and oncological outcomes of robot-assisted laparoscopic radical cystectomy (RARC) to open RC (ORC) at a single institution. PATIENTS AND METHODS: A retrospective analysis was performed on a consecutive series of patients undergoing RC (100 RARC and 100 ORC) at Wake Forest University with curative intent from 2006 until 2010. Complication data using the Clavien system were collected for 90 days postoperatively. Complications and other perioperative outcomes were compared between patient groups. RESULTS: Patients in both groups had comparable preoperative characteristics. The overall and major complication (Clavien >/= 3) rates were lower for RARC patients at 35 vs 57% (P = 0.001) and 10 vs 22% (P = 0.019), respectively. There were no significant differences between groups for pathological outcomes, including stage, number of nodes harvested or positive margin rates. CONCLUSION: Our data suggest that patients undergoing RARC have perioperative oncological outcomes comparable with ORC, with fewer overall or major complications. Definitive claims about comparative outcomes with RARC require results from larger, randomised controlled trials.
Discovery of novel second mitochondria-derived activator of caspase mimetics as selective inhibitor of apoptosis protein inhibitors.[Pubmed:24623800]
J Pharmacol Exp Ther. 2014 May;349(2):319-29.
Inhibitor of apoptosis (IAP) proteins are widely considered as promising cancer drug targets, especially for drug-resistant tumors. Mimicking the IAP-binding motif of second mitochondria-derived activator of caspases (SMAC) is a rational strategy to design potential IAP inhibitors. In this report, we used the bioactive conformation of AVPI tetrapeptide in the N terminus of SMAC as a template and performed a shape-based virtual screening against a drug-like compound library to identify novel IAP inhibitors. Top hits were subsequently docked to available IAP crystal structures as a secondary screening followed by validation using in vitro biologic assays. Four novel hit compounds were identified to potently inhibit cell growth in two human melanoma (A375 and M14) and two human prostate (PC-3 and DU145) cancer cell lines. The best compound, UC-112 [5-((benzyloxy)methyl)-7-(pyrrolidin-1-ylmethyl)quinolin-8-ol], has IC50 values ranging from 0.7 to 3.4 microM. UC-112 also potently inhibits the growth of P-glycoprotein (P-gp)-overexpressed multidrug-resistant cancer cells, strongly activates caspase-3/7 and caspase-9 activities, and selectively downregulates survivin level at a concentration as low as 1 microM. Coincubation of UC-112 with a known proteasome inhibitor Z-Leu-Leu-Leu-CHO (MG-132) rescued survivin inhibition, consistent with the anticipated mechanism of action for UC-112. As a single agent, UC-112 strongly inhibits tumor growth and reduces both X chromosome-linked IAP and survivin levels in an A375 human melanoma xenograft model in vivo. Overall, our study identified novel scaffolds, especially UC-112, as new platforms on which potent and selective IAP antagonists can be developed.


